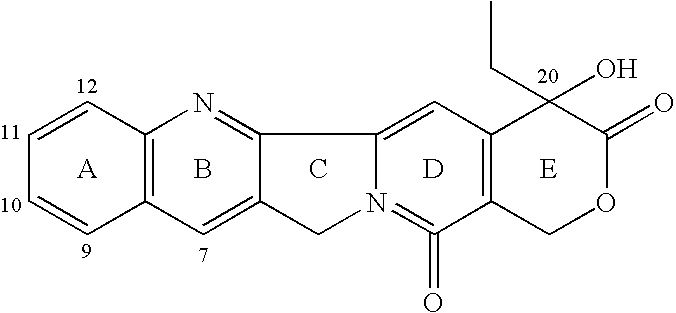
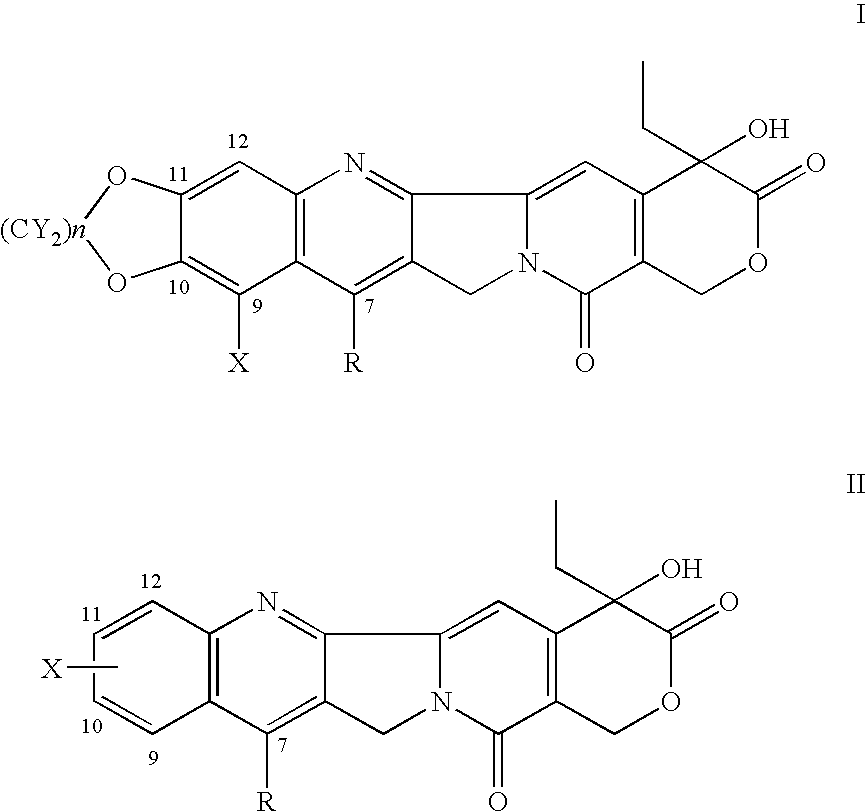
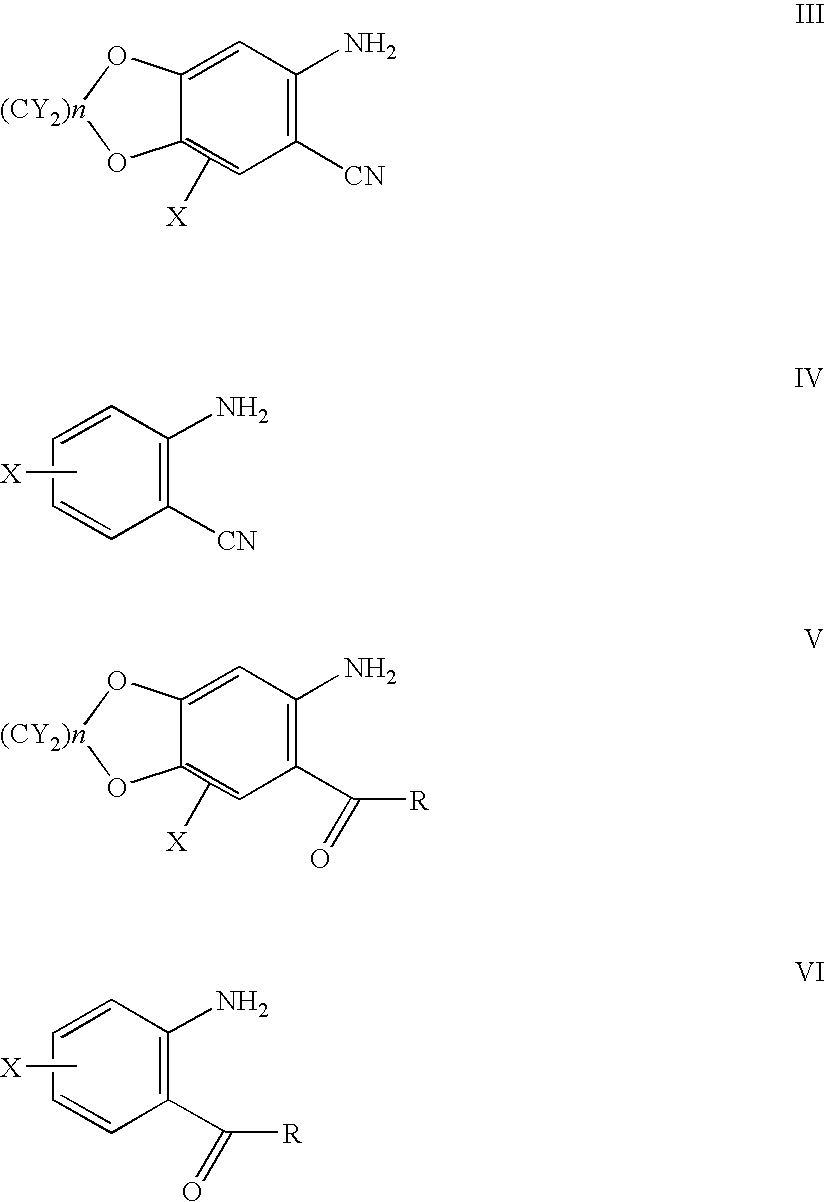
7-Substituted camptothecin and camptothecin analogs and methods for producing the same
a technology of camptothecin and substituted camptothecin, which is applied in the field of preparing 7substituted camptothecin compounds and 7substituted camptothecin analogs, can solve the problems of poor yield of csub>4/sub>, csub>5/sub>, and achieve excellent yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
2-Amino-4,5-methylenedioxy-phenylbenzylmethanone
[0080] To a stirred solution of 1.5 g (10.0 mmol) of 2-amino-4,5-methylenedioxy-benzonitrile in THF (40 mL) was added CuBr (50 mg, 0.34 mmol) and benzylmagnesium chloride (40 mL, 1.0 M solution in Et2O). The reaction mixture was refluxed for 12 h. After cooling to 25° C., H2O (5 mL) was added followed by 15% H2SO4 (15 mL). After stirring for 14 h, ether (50 mL) was added. Organic layer was separated. Aqueous layer was extracted with ether (2×50 mL). The combined organic layer was dried and evaporated. Following chromatography (silica gel, CHCl3), 1.2 g (52%) of the title compound was obtained. IR (CHCl3) 1675 cm−1 MS m / z 255 (M+).
example 2
7-Benzyl-10, 11-MD-20(S)-Camptothecin
[0081] A mixture of S-tricyclic ketone (1.0 g, 4.2 mmol), 2-amino-4,5-methylenedioxy-phenylbenzylmethanone (1.1 g, 4.3 mmol) acetic acid (1 mL), p-TsOH (50 mg) in toluene (100 mL) was refluxed for 15 h. After removing the solvent, the crude product was purified by column chromatography (silica gel, CHCl3) to yield the product as a cream powder (1.33 g, 66%)1H-NMR (DMSO-d6) δ 0.89 (t, 3H), 1.91 (m, 2H), 4.62 (s, 2H), 5.22 (s, 2H), 5.41 (s, 2H), 6.10 (s, 2H), 6.50 (s, 1H), 6.90-7.10 (m, 5H), 7.21 (s, 1H), 8.07 (s, 1H), 8.22 (s, 1H); MS: m / z 483 (M+1)+.
example 3
7- Trimethylsilylmethyl-10,11 -MD-20(S)-Camptothecin
[0082] The title compound was prepared following analogous procedures as described in Examples 1 and 2 and involving the use of trimethylsilyl magnesium chloride as the Grignard reagent. 1H-NMR (DMSO-d6+CDCl3) δ 0.87 (t, 3H), 1.83 (m, 2H), 2.28 (s, 2H), 5.11 (s, 2H), 5.37 (s, 2H), 6.25 (s, 2H), 6.47 (s, 1H), 7.20 (s, 1H), 7.43 (s, 1H), 7.48 (s, 1H). MS m / z 478 (M+).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Electrical conductance | aaaaa | aaaaa |
| Density | aaaaa | aaaaa |
| Density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com