Low dose haptenized tumor cell and tumor cell extract immunotherapy
a tumor cell and immunotherapy technology, applied in the field of low dose haptenized tumor cells and tumor cell extract immunotherapy, can solve the problems of not reaching 100%, not being established that these haptens will be effective in humans, etc., and achieve the effect of more effective treatment regimens
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Treatment Protocol for Melanoma Patients
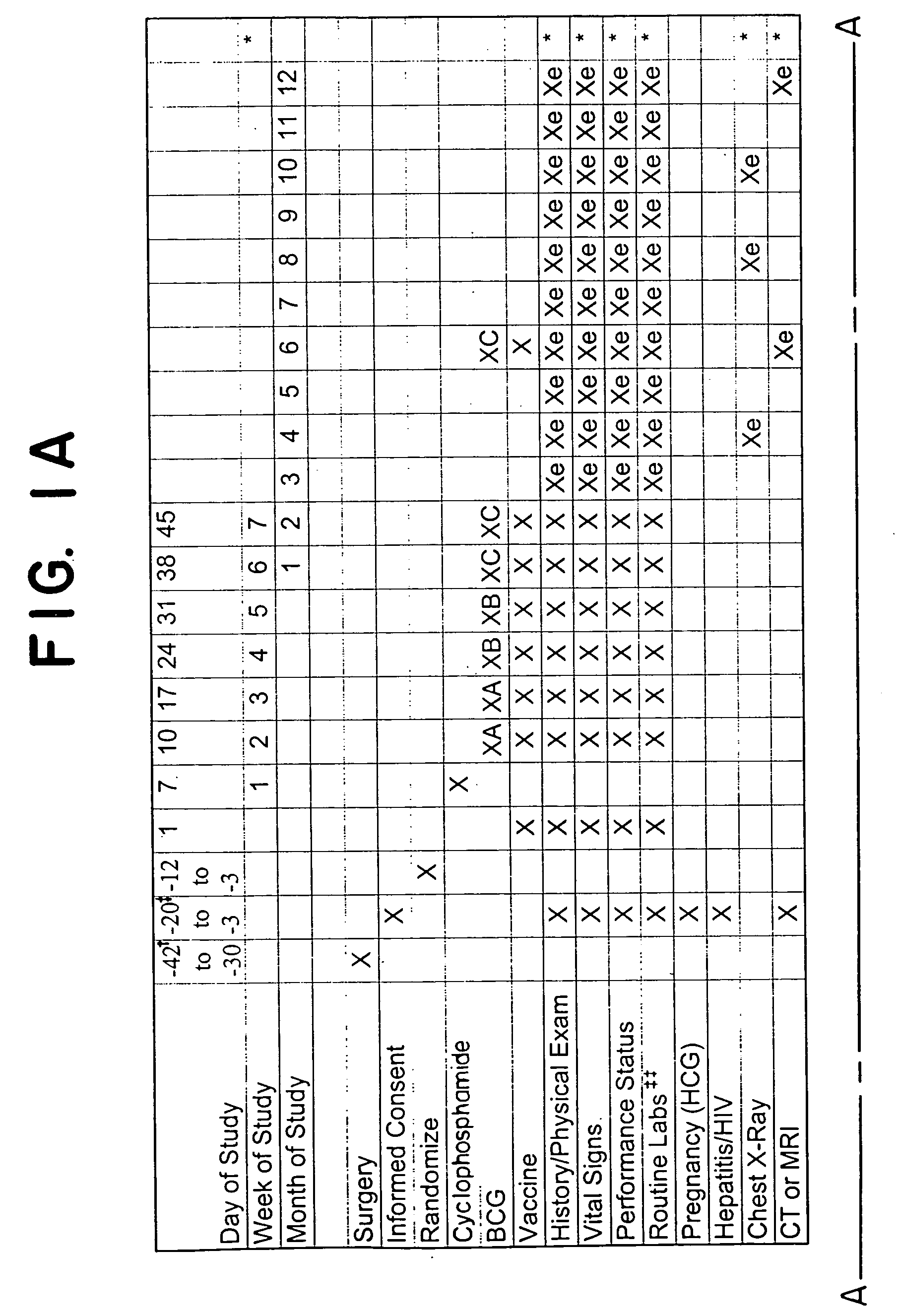
[0095] Patient group. The treatment efficacy of DNP-modified autologous melanoma cells (vaccine) is studied in post-surgical stage III melanoma patients having metastatic lymph node involvement, or stage IV melanoma patients with lung metastases. Prior to the initiation of the vaccine treatment, preferably within two months from the starting point, one or more tumor masses are surgically resected from each patient. For axillary nodes, a formal axillary node dissection is performed. For inguinal nodes, a superficial inguinal dissection is performed, with a deep dissection at the discretion of the surgeon. For other lymph node-bearing areas, an appropriate node dissection is performed. An adequate tumor sample, approximately 2 cm or 5 g, yielding at least about 50×106 cells, is kept under sterile conditions to be used for vaccine preparation. Patients are preferably confirmed melanoma-free by computed tomography (CT) or magnetic resonance imagi...
example 2
Pretreatment with BCG-free Haptenized Tumor Cells Improves Therapeutic Outcome
[0101] Anti-metastatic effects are induced by DNP-modified, irradiated, autologous tumor cell vaccine (preceded by low-dose cyclophosphamide) in a newly developed animal model, (described in co-pending application Ser. No. 60 / 180,257, attorney docket No. 1225 / 0G680, filed on Feb. 4, 2000). This animal model, particularily useful for providing information on the effectiveness of postsurgical immunotherapies for recurrence of metastatic disease, was used to evaluate whether a modified protocol could offer even greater therapeutic benefits. Specifically, treatment regimen (A), consisting of low-dose cyclophosphamide followed by unmodified or DNP-modified autologous (syngeneic) tumor cell vaccine, was compared to a new pretreatment regimen (B), in which a single dose of autologous DNP-modified tumor cells (free of BCG) was administered prior to initiation of the low-dose cyclophosphamide treatment, followed b...
example 3
The Timing of an Haptenized or Non-Haptenized, Irradiated, Autologous, Melanoma, Tumor Cell Treatment Prior to Administration of an Immunomodulator and an Haptenized Vaccine Improves Therapeutic Outcome
[0111] The anti-metastatic effects of hapten-modified vaccines are increased by the administration of a “priming” induction dose administered several days before administration of a low dose of the immunomodulator cyclophosphamide (CY) and DNP vaccine mixed with BCG. The induction (or “priming”) dose comprised of one of the three following compositions: (1) 106 irradiated, autologous, tumor cells (AU TC); (2) a mixture of irradiated, autologous, tumor cells and irradiated, autologous, DNP-modified tumor cells 106 of each; or (3) 106 irradiated, autologous, DNP-modified tumor cells.
Material and Methods
[0112] Patient group. The treatment efficacy of administering a “priming” induction dose of haptenized or non-haptenized or a mixture of haptenized and non-haptenized, irradiated, auto...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com