Enhancing the circulating half-life of antibody-based fusion proteins
a fusion protein and circulating half-life technology, applied in the field of fusion proteins, can solve the problems of limited use of recombinantly-produced antibody-based fusion proteins, achieve enhanced in vivo circulating half-life, decrease and increase the interaction of fc moiety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Construction of Antibody-IL-2 Genes with Substitutions of the Lys Codon at the Fusion Junction
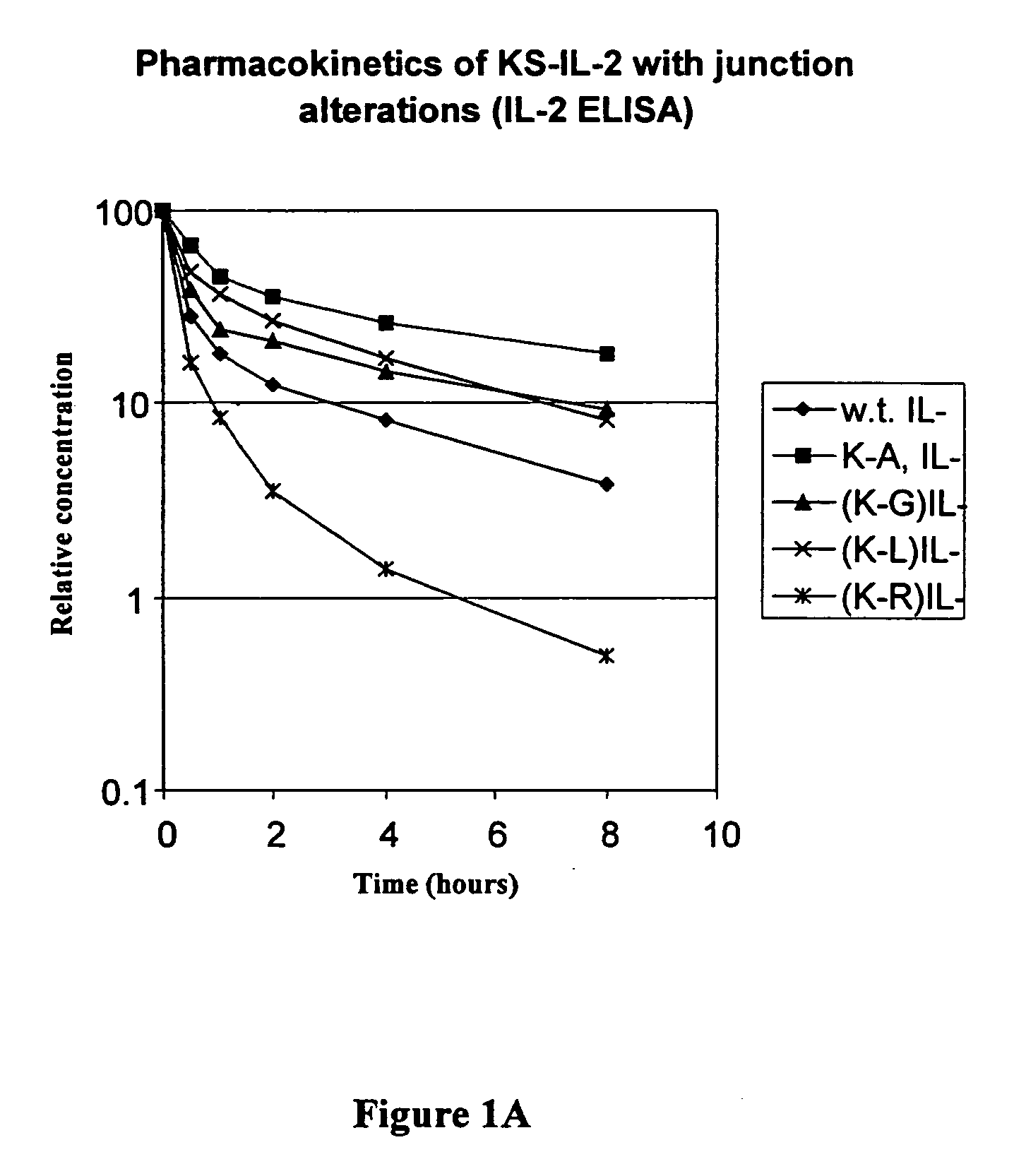
[0057] The amino acid sequence at the junction of the antibody-IL-2 fusion protein is SerProGlyLys-AlaProThr (SEQ ID NO: 1), in which the SerProGlyLys (SEQ ID No. 2) is the normal carboxy terminus of the heavy chain of the antibody, and AlaProThr is the N-terminal sequence of mature IL-2. In order to determine the effect alterations in the region of the fusion junction on the pharmacokinetics of the fusion protein, substitutions or deletion of the residue were made by mutagenesis, as described below.
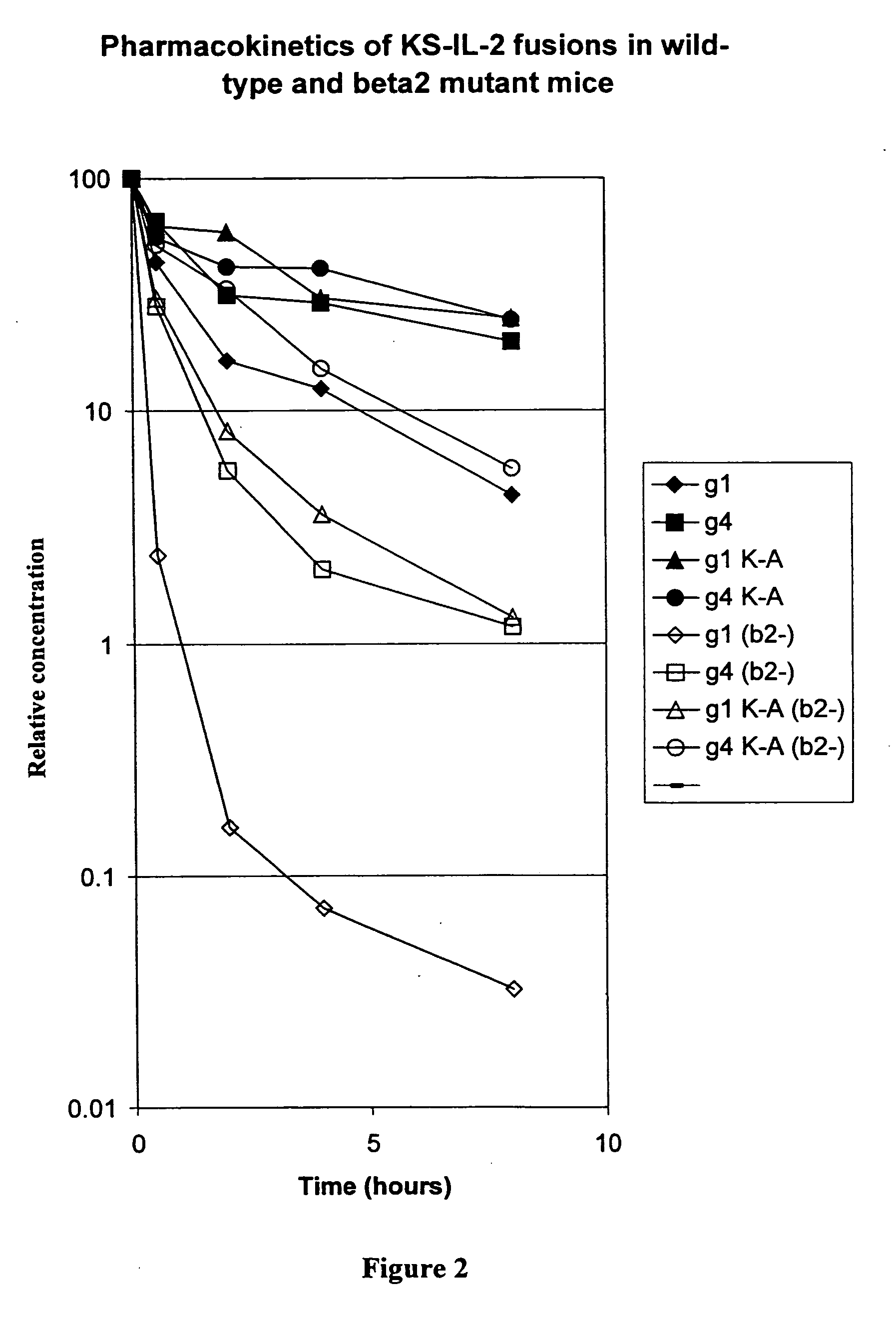
[0058] The expression vector for immunocytokines was described in Gillies at al., (1998) J. Immunol. 160:6195-6203. In the human gamma-1 gene encoding the heavy chain, the XmaI restriction site located 280 bp upstream of the translation stop codon was destroyed by introducing a silent mutation (TCC to TCA). Another silent mutation (TCT to TCC) was introduced to the Ser codon three residues ups...
example 2
Construction of Antibody-IL-2 Genes Encoding Extra Amino Acid Residues at the Fusion Junction
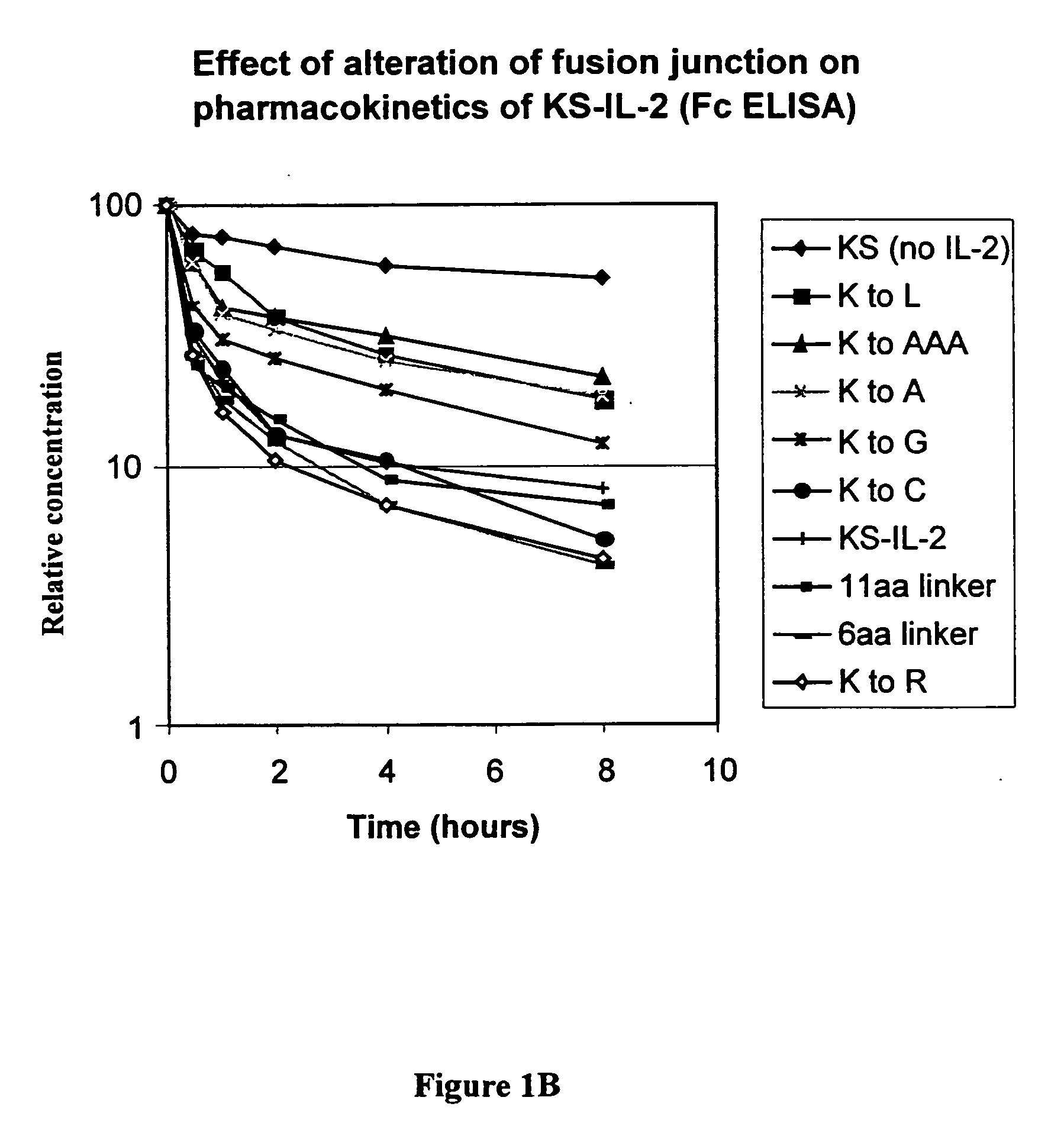
[0069] It is common in the art to separate domains in fusion proteins with flexible linkers containing amino acid residues such as glycine and serine. The importance of the spacing between the CH3 and IL-2 was studied in the following mutagenesis experiments. Blunt ended oligonucleotide duplexes encoding different number of amino acid residues were inserted into the SmaI endonuclease restriction site (same recognition site as the XmaI mentioned above) of the huKS-IL-2 expression vector by ligation; and the correct orientation of insertion was confirmed by DNA sequencing. As discussed above, oligonucleotide duplexes with 5′-hydroxyl ends were used to eliminate self ligation.
9.) Lys to Cys Substitution with Linker Ligation
[0070] The following linker (oligonucleotide duplex) was inserted into the SmaI endonuclease restriction site of the huKS-IL-2 expression vector by ligation. The sequence...
example 3
Construction of Antibody-IL-2 Genes with Substitutions of the Pro Codon at the Fusion Junction
[0074] The proline in the sequence ProGlyLys at the carboxyl terminus of CH3 is mutated to Ala, Leu or Gly, and other amino acids. This is accomplished by replacing a 25 base-pair SapI-SmaI fragment of the KS-IL-2 expression vector by an oligonucleotide duplex encoding the desired change. Each of the following oligonucleotide duplexes has a SapI cohesive end (3-base overhang) and a blunt end (for ligating to the SmaI end of the restriction fragment). The substitutions at the Pro codon are denoted in bold. These substitutions had no significant effect on the pharmacokinetics of the fusion protein, indicating that the structural properties of the Pro residue have no significant effect on the pharmacokinetics of the fusion protein
12.) Pro to Ala Substitution(SEQ ID NO:28)5′ CG CAG AAG AGC CTC TCC CTG TCC GC 3′(SEQ ID NO:29)3′ TC TTC TCG GAG AGG GAC AGG CG 5′13.) Pro to Leu Substitution(...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com