Methods for staining cells for identification and sorting
a cell and staining technology, applied in nanoinformatics, instruments, veterinary instruments, etc., can solve the problems of complex cells that outweigh the advantages, fixation procedures often incur artifacts, and damage to the rest of the cell structure, so as to facilitate the introduction of dye and enhance sperm viability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
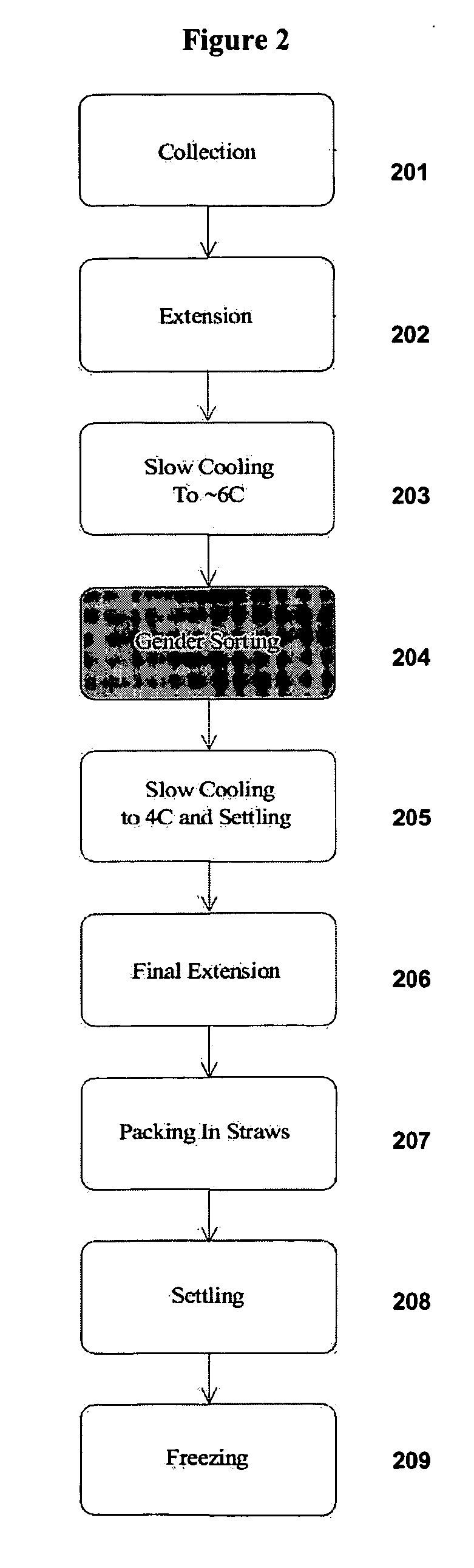
example 1
In vivo Staining of Sperm Cells with a DNA-Specific Dye
[0082] Methods and Materials
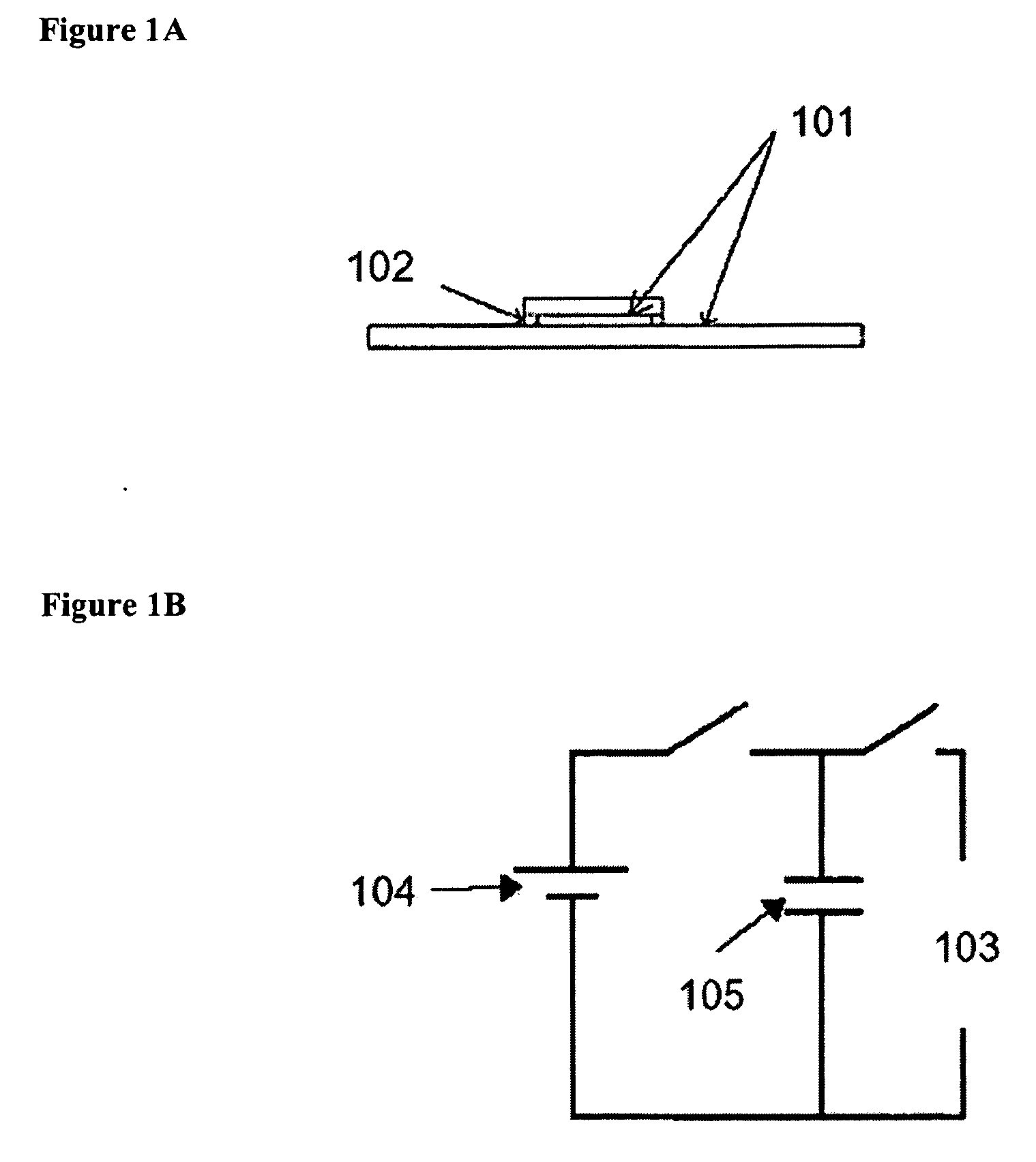
[0083] Electroporation unit A sample cell was formed by two parallel glass slides coated with 1,500-2000 angstroms (Å) of indium tin oxide (ITO). The slides were separated by fragments of number zero glass cover slips, yielding a slide separation of 100 millimeters (mm).
[0084] The sample cell was connected to a resistor-capacitator (RC) circuit by alligator clips. A direct current (DC) power supply was used to charge a capacitor. When a switch was thrown, the discharging capacitor generated a time-dependant and spatially uniform electric field across the sample. An oscilloscope was used to monitor the voltage across the sample cell as a function of time.
[0085] The RC circuit formed by the sample cell and capacitor allowed for a well-controlled electric field to be generated. The resistance (R) of the circuit was left floating—that is, determined by the geometry and content of the sample cell. Typi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com