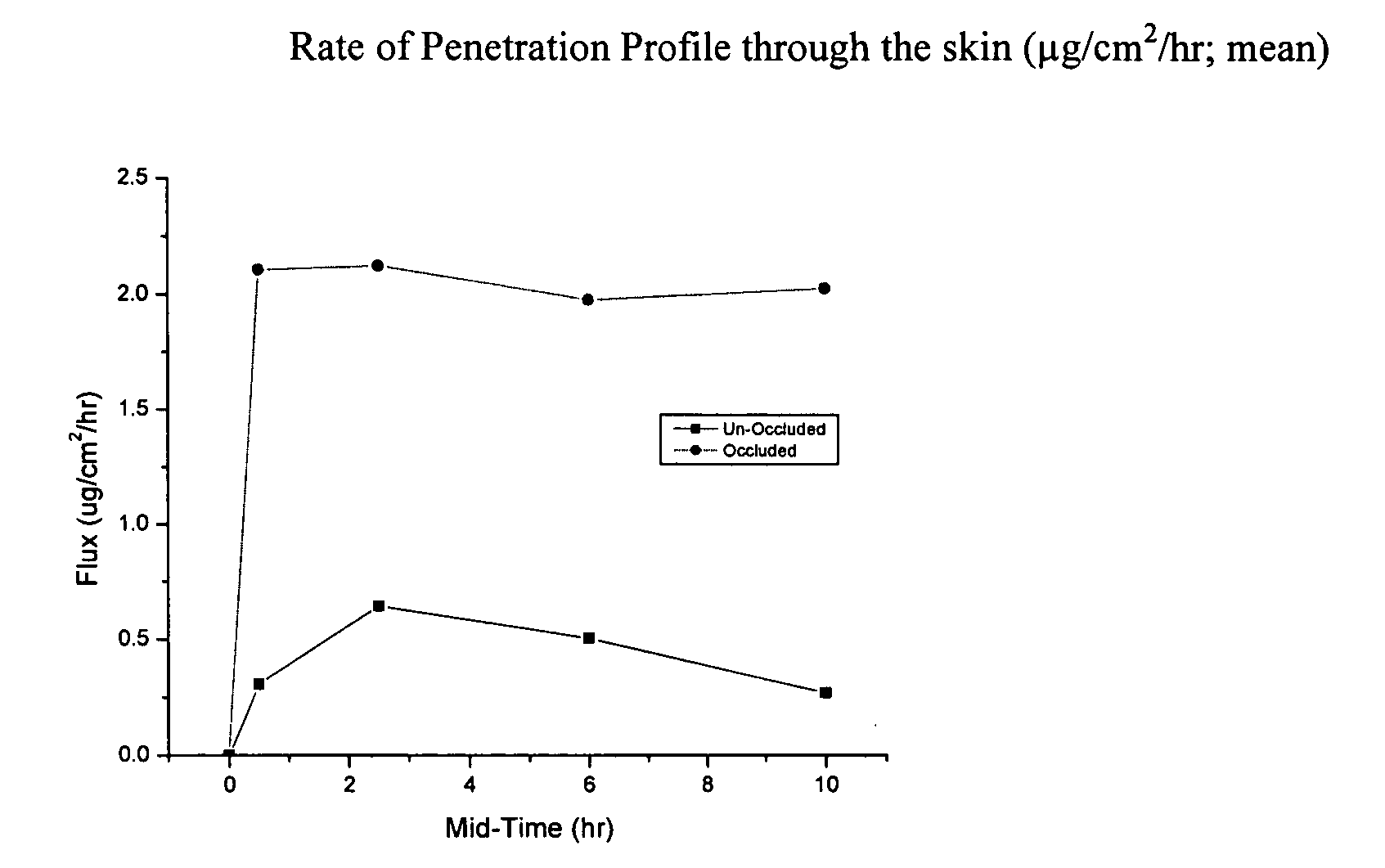
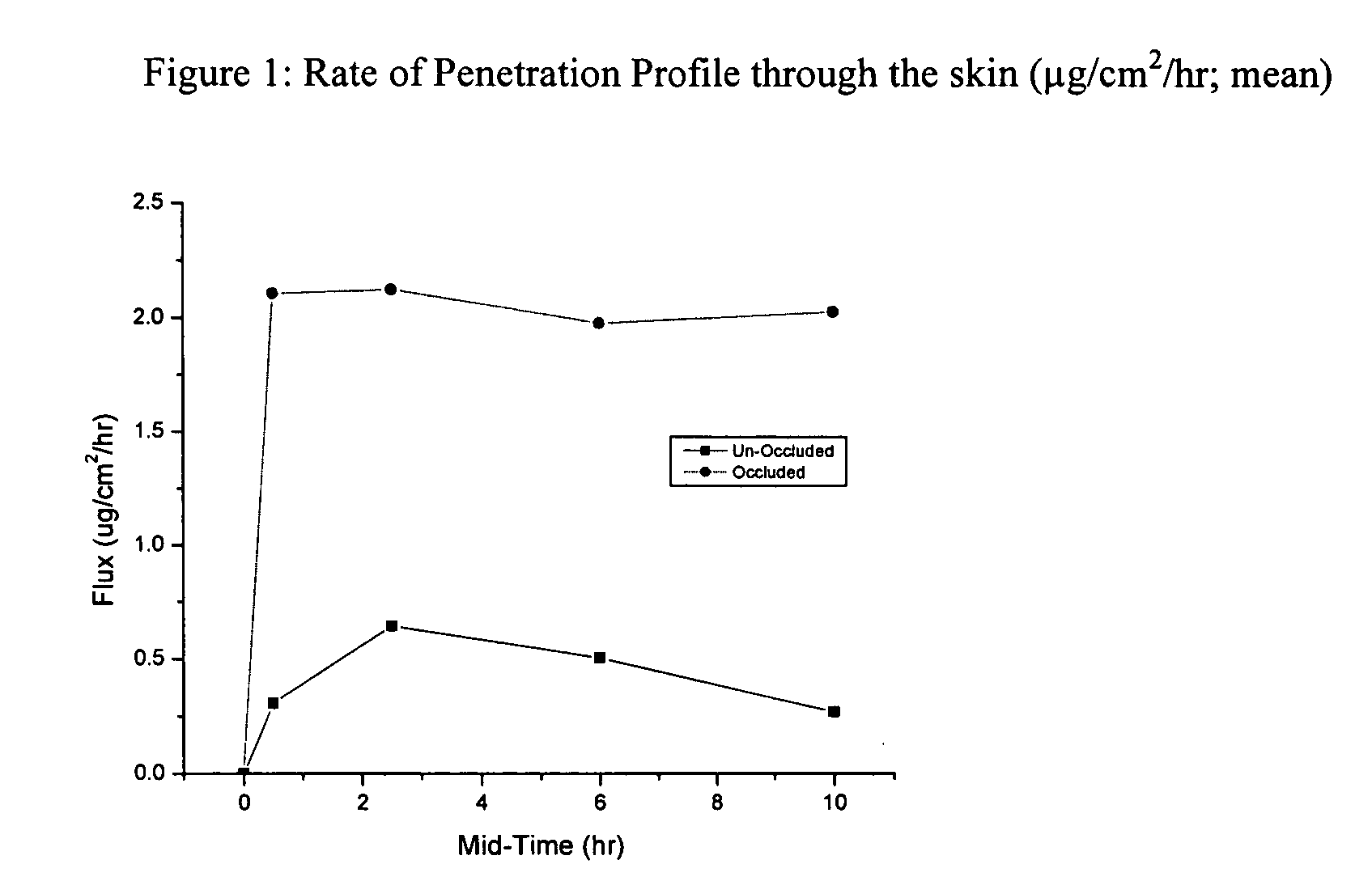
Microbial cellulose materials for use in transdermal drug delivery systems, method of manufacture and use
a technology of microbial cellulose and transdermal drug delivery, which is applied in the field of polysaccharide materials, can solve the problems of unrecognized skin, unrecognized skin, and no method previously described suggests creating a highly hydrated environmen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Delivery of Antibiotics
[0054] In cases of difficult to heal wounds, clinicians believe that there may be microbial colonization that has not reached levels of infection. Depending on the type of microbe (gram negative or gram positive bacteria, fungus, etc) a specific treatment is optimal. Patients having wounds that were no longer responsive to treatment were evaluated for colonization. Those with gram-positive bacteria were treated with microbial cellulose that had been soaked in a solution of vancomycin. Dressings were changed as needed. Results indicated that the vancomycin treated wounds improved and went on to heal in an average of five weeks. Serum trough levels of the antibiotics (values of the amount of an antibiotic that has passed through the skin and into the blood) demonstrated a profound local effect. Minute quantities of vancomycin were noted in the circulatory system. This demonstrated the delivery of antibiotics by microbial cellulose.
example 2
Delivery of Lidocaine by Microbial Cellulose
[0055] A solution of 4% lidocaine was added to microbial cellulose and allowed to soak into the microbial cellulose for 5 minutes. This was then placed onto the patient's wound for 5 to 15 minutes prior to sharp debridement of necrotic tissue. Delivery of the lidocaine through the thick layer of dry necrotic tissue was evidenced by the lack of pain that the patient felt during the debridement process. A similar study performed on intact skin demonstrated that lidocaine was released over a 7-day period to relieve pain administered by a sharp object.
example 3
Delivery of Thrombin by Microbial Cellulose
[0056] Thrombin was reconstituted per instructions for use and applied to microbial cellulose for 5 minutes. The dressing containing the thrombin solution was applied to a patient that had excessive diffuse bleeding after sharp debridement and was removed after 24 hours. The combination of thrombin and microbial cellulose effectively established hemostasis.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
| mechanical | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com