Method and device for abrading skin
a technology of skin absorption and skin absorption, which is applied in the field of skin absorption methods and devices, can solve the problems of excessively reducing the skin barrier function, increasing the risk of infection, and pain, and achieves the effects of minimal irritation, simple and reliable manner, and efficient penetration of stratum corneum
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
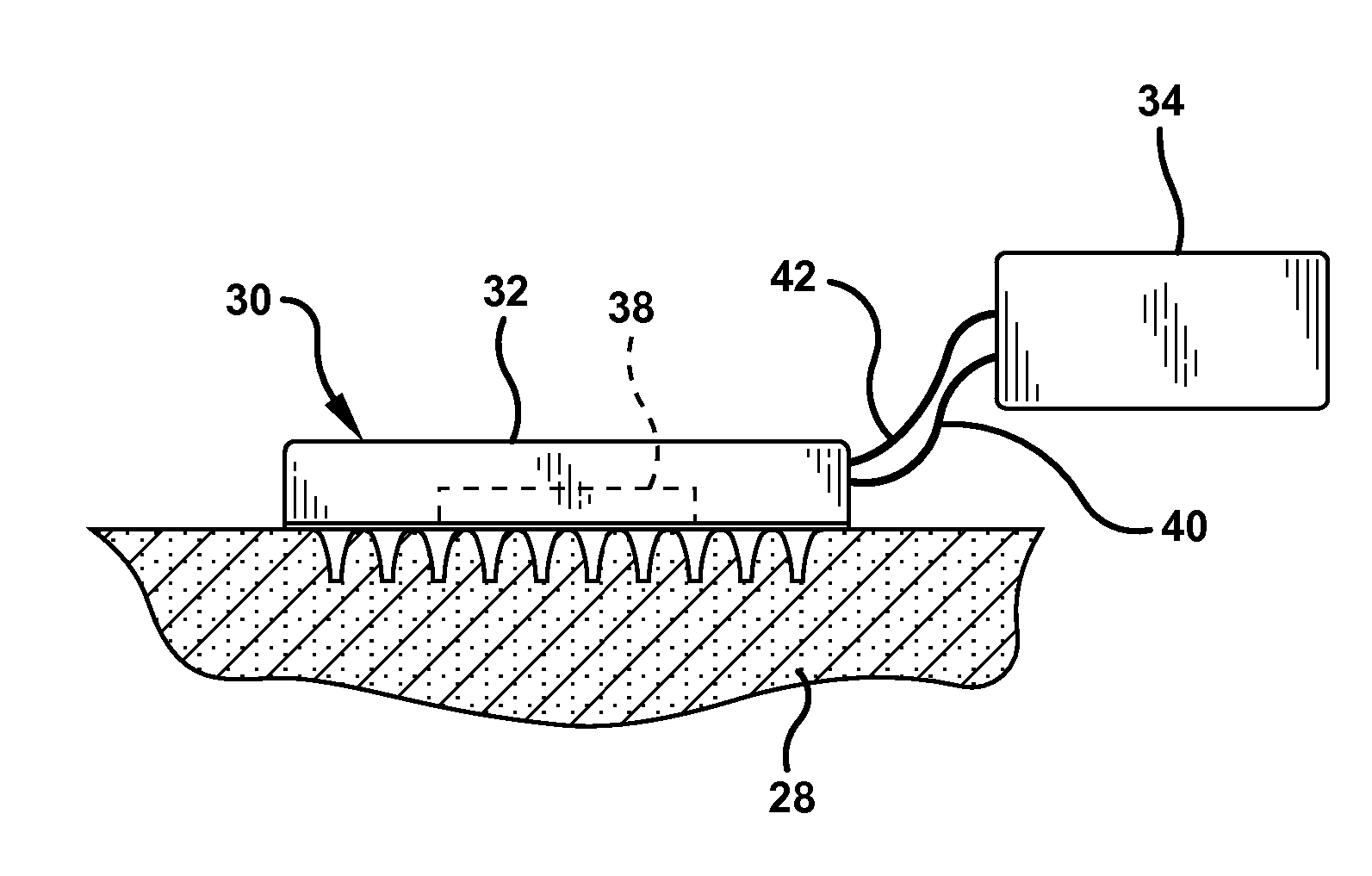
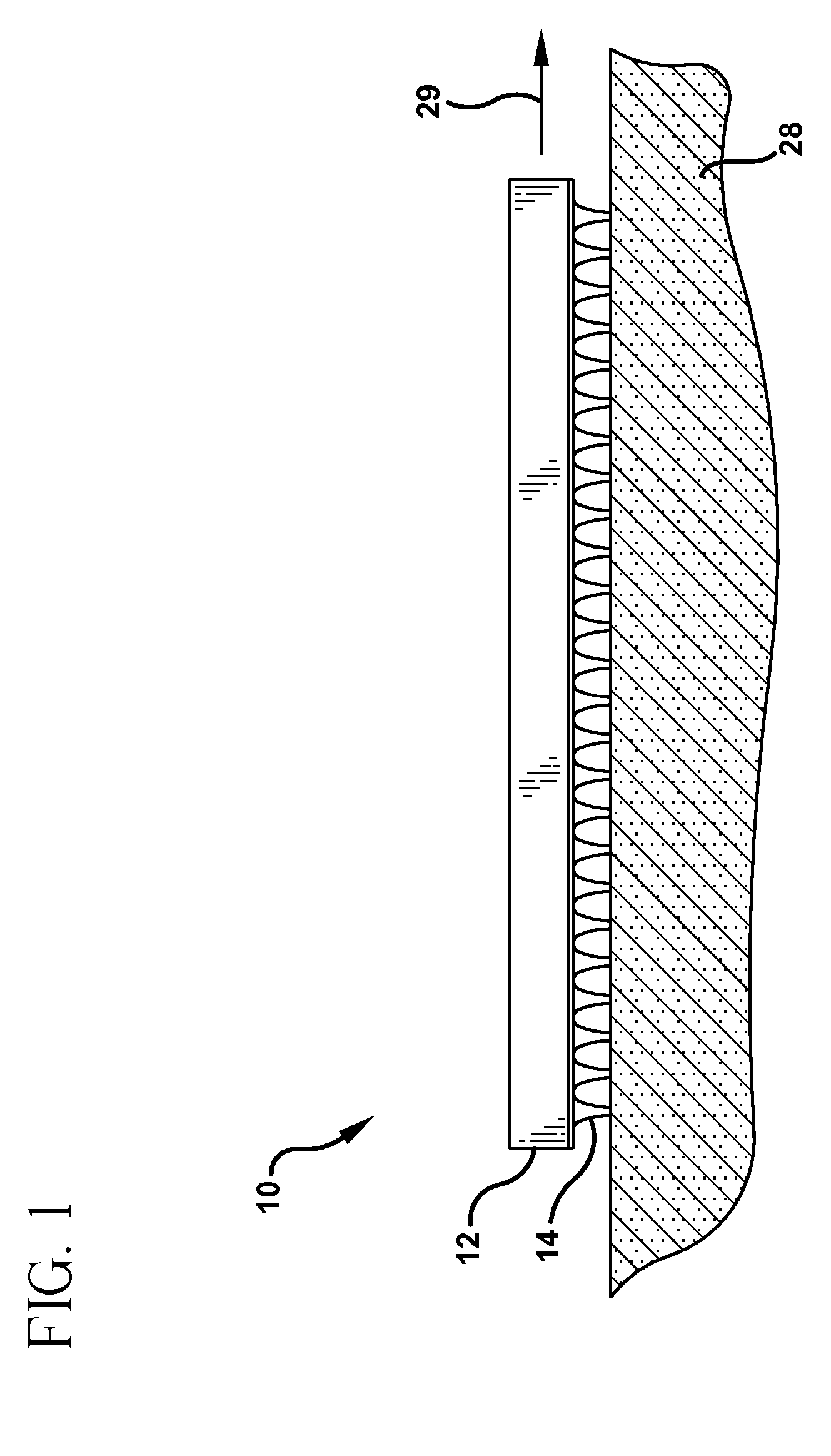
[0077] A microabrader having a surface area of about 1 cm2 is provided with a plurality of microneedles having a length of about 250 microns. The microneedles were arranged in a plurality of uniform rows and columns to provide a needle density of about 200 needles per cm2.
[0078] The microabrader was gently placed on the back of guinea pigs and moved across the skin to produce an abraded area of about 4 cm2. The microabrader was scraped along the same path several times to produce an abraded delivery site. The microabrader was removed and a commercially available anesthetic cream sold under the trademark EMLA was applied. The anesthetic cream was applied to a second group of guinea pigs in the same location without abrading.
[0079] The topical anesthetic was allowed to contact the skin for one hour before conducting the test for anesthesia. Each guinea pig received five controlled stimuli on the treatment site. In the negative control group, the test site was defined by a similar ci...
example 2
[0082] A microabrader having microneedles of about 200 microns in length was used to abrade the skin of guinea pigs in preparation for delivery of the anesthetic lidocaine by iontophoresis.
[0083] Iontophoresis patches were applied to the abraded delivery site to deliver lidocaine for 5 minutes at 1.8 mA. The control delivery sites without abrasion were treated with an identical lidocaine iontophoresis device for 5 minutes. The anesthesia obtained by the twitch method of Example 1 is presented in the graph of FIG. 8. The iontophoresis current was discontinued after 5 minutes and the extent of anesthesia measured for 1 hour. As shown by the data of FIG. 8, iontophoresis applied to a microabrasion site attained 100% anesthesia immediately after application, while the same iontophoresis without abrading attained about 50% anesthesia.
[0084] As shown in the graph of FIG. 8, the abraded site maintained a higher percent anesthesia than the site without abrasion.
example 3
[0085] This example evaluates the dose of lidocaine in the tissue. Lidocaine iontophoresis was conducted on anesthetized Yorkshire pigs using patches spiked with 14C lidocaine. Four abraders were selected having different microneedle lengths and shapes as follows: 100 microns with sharp points; 100 microns with blunt, flat tips; 200 microns with sharp points; and 200 microns with blunt, flat tips.
[0086] A delivery site was prepared on the pigs by abrading the skin with each of the microabraders and the patches were applied at about 1.8 mA. The radiolabeled lidocaine that was delivered to the pig was imaged on tape strips and assayed in the skin underlying the patch application site. The tape strips qualitatively show enhancement of lidocaine delivery with abrasion.
[0087] The treated skin was biopsied and cut into sections that were then dissolved and assayed for radiolabeled lidocaine with liquid scintillation counting. The average doses were determined by averaging the tissue dos...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com