Implantable vascular graft
a vascular graft and implantable technology, applied in the field of endoluminal vascular prosthesis, can solve the problems of high mortality, large risk, and rupture of the sac, and achieve the effect of reducing migration and risk of endoleaks
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
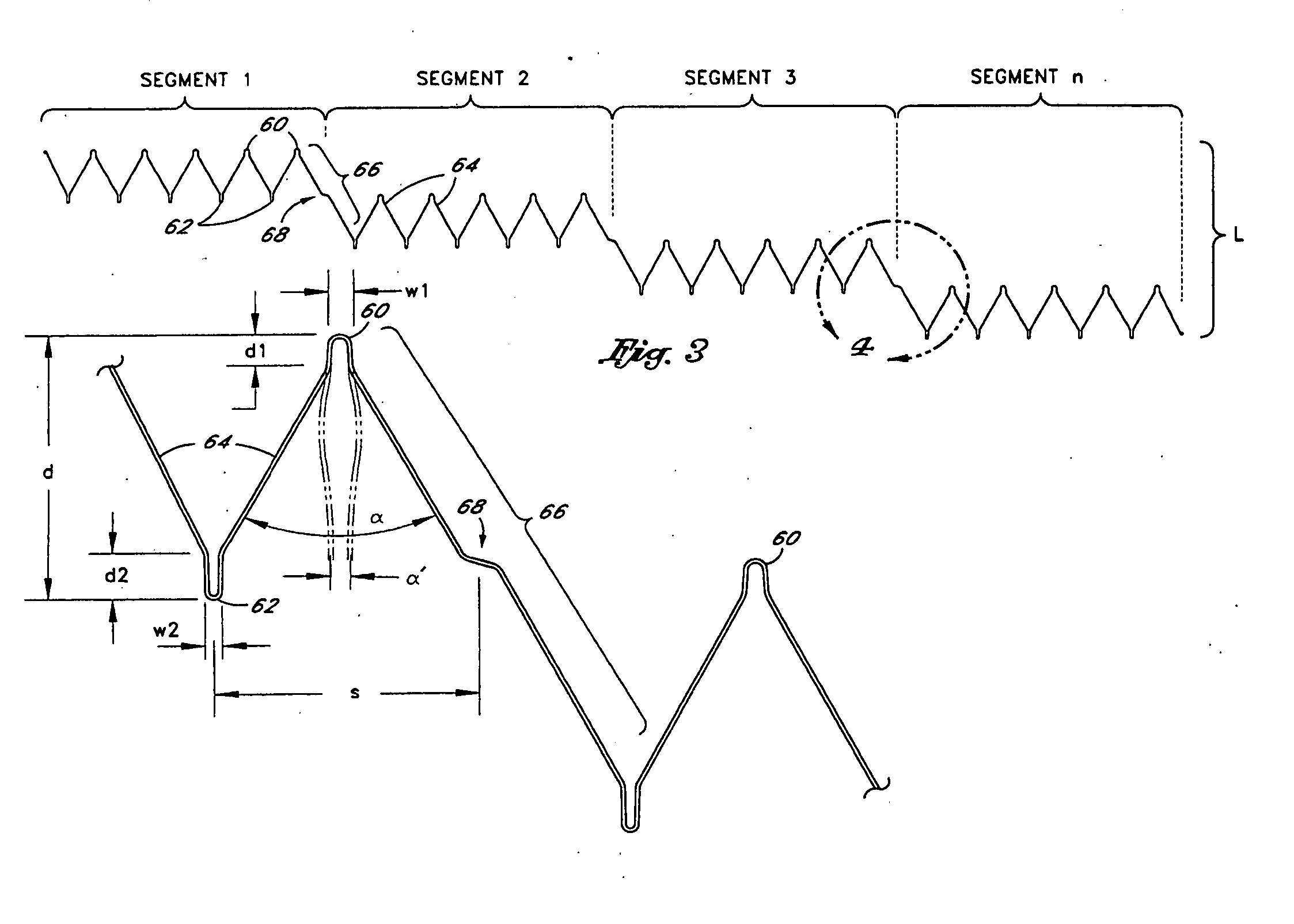
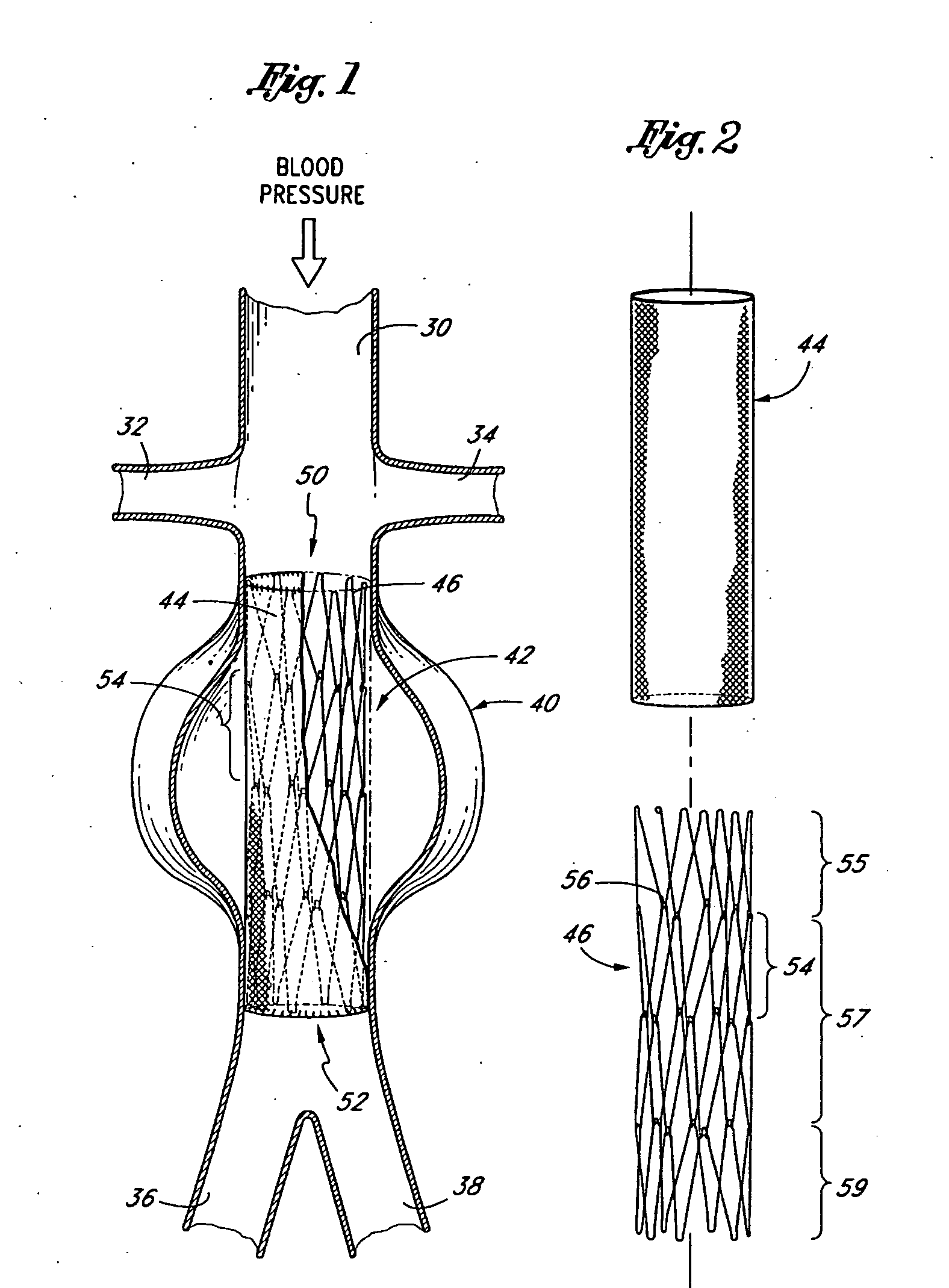
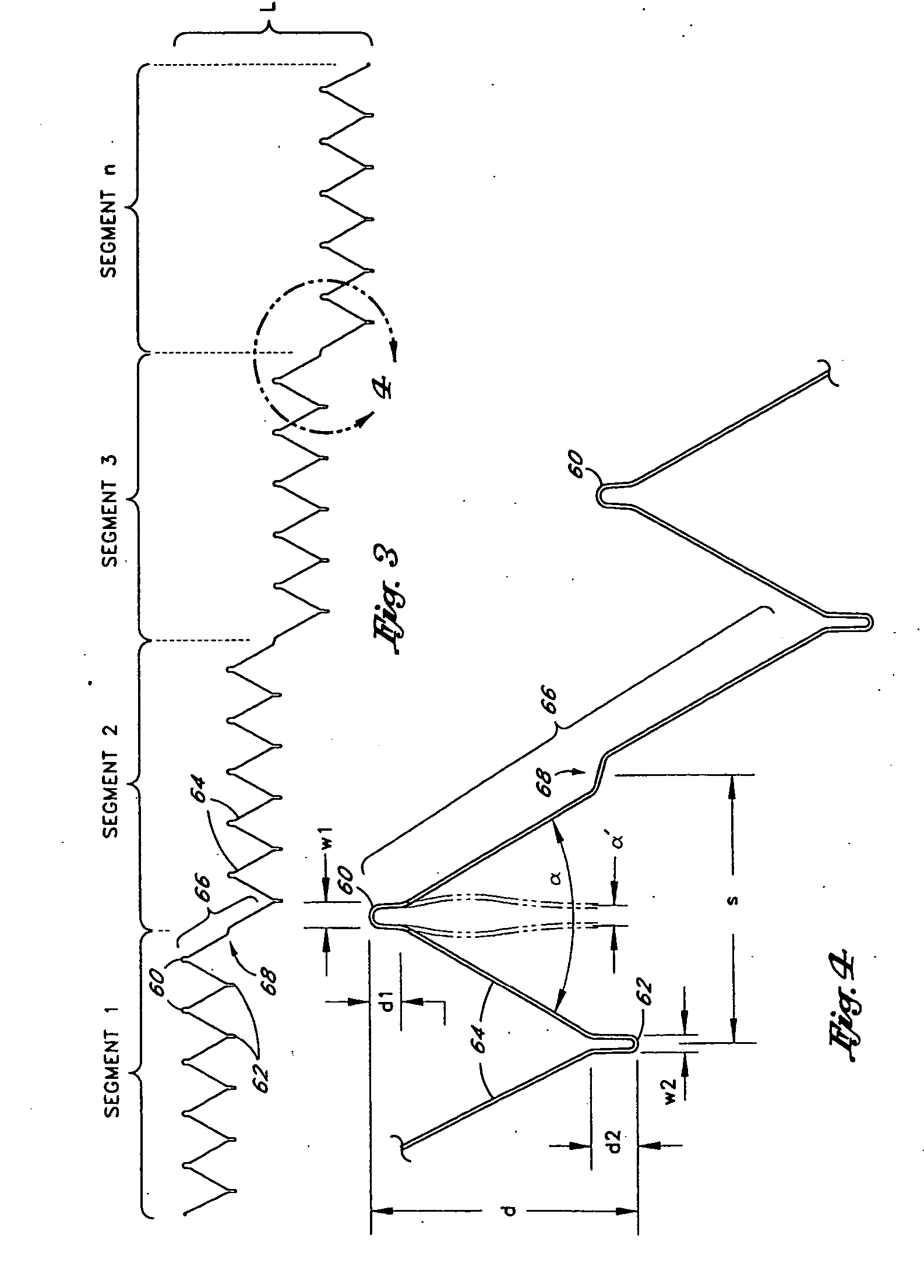
[0073] Referring to FIG. 1, there is disclosed a schematic representation of the abdominal part of the aorta and its principal branches. In particular, the abdominal aorta 30 is characterized by a right renal artery 32 and left renal artery 34. The large terminal branches of the aorta are the right and left common iliac arteries 36 and 38. Additional vessels (e.g., second lumbar, testicular, inferior mesenteric, middle sacral) have been omitted for simplification. A generally symmetrical aneurysm 40 is illustrated in the infrarenal portion of the diseased aorta. An expanded straight segment endoluminal vascular prosthesis 42, in accordance with the present invention, is illustrated spanning the aneurysm 40.
[0074] The endoluminal vascular prosthesis 42 includes a polymeric sleeve 44 and a tubular wire support 46, which are illustrated in situ in FIG. 1. The sleeve 44 and wire support 46 are more readily visualized in the exploded view shown in FIG. 2. The endoluminal prosthesis 42 i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com