Methods and compositions for the reduction of neutrophil influx and for the treatment of bronchpulmonary dysplasia, respiratory distress syndrome, chronic lung disease, pulmonary fibrosis, asthma and chronic obstructive pulmonary disease
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Administration of rhCC10 to Premature Infants
[0051] Patients were enrolled in a placebo-controlled, blinded, dose ranging study at four hospital sites.
[0052] rhCC10 was produced in E. coli bacteria and purified by a proprietary process (Claragen, Inc., College Park, Md.), described in U.S. Application Publication Nos. US 2003-0109429 and US 2003-0207795, both of which are incorporated by reference in their entirety. The protein for the study was provided as a >98% pure solution of recombinant human CC 10 homodimer. The biological activity of each batch was compared using a proprietary secretory PLA2 inhibition assay, described in U.S. Application Publication Nos. US 2002-0169108 which is incorporated herein by reference.
[0053] Newborn infants who met the following criteria were enrolled: 1) age ≦24 h; 2) birth weight between 700 and 1,300 g; 3) gestational age ≧24 wk; 4) diagnosis of RDS based on clinical and radiographic criteria; 5) requirement for intubation and mechanical ven...
example 2
TAF Concentrations of CC10 in Patients Treated with rhCC10
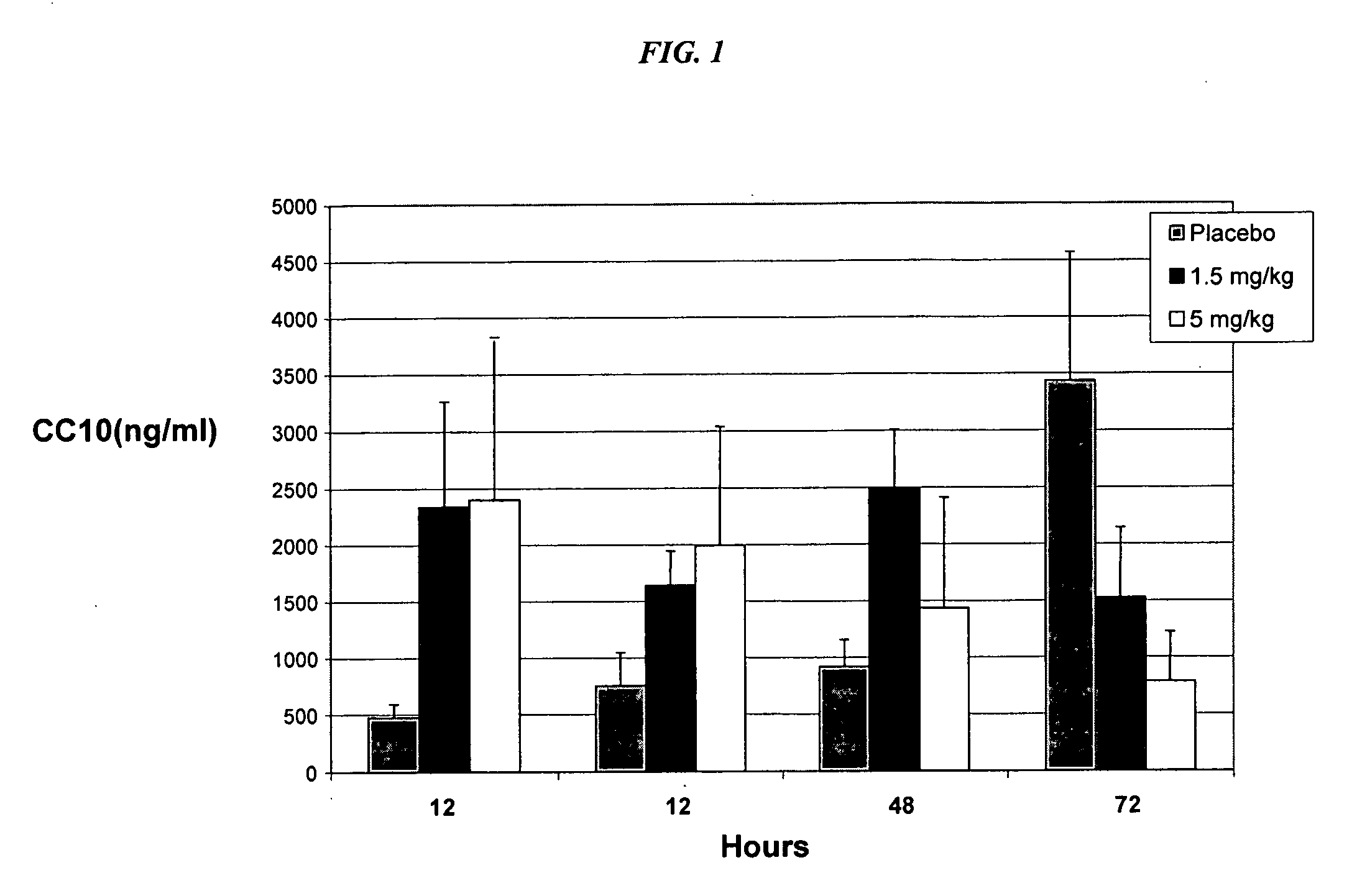
[0061] With reference to FIG. 1 it has been found that during the first 48 hours of life, after an initial dose of rhCC10, significantly increased overall CC10 concentration occurred in patients receiving rhCC10 in dosages comprising either 1.5 mg / kg of body mass or 5 mg / kg of body mass versus placebo. Thus, administration of rhCC10 during the first 24 hours of life has a significant positive impact on CC10 levels in patients during the first two days of life. Furthermore, administration of rhCC10 will increase overall CC10 concentrations in patients.
[0062] Reference is now made to Table 2, as well as to FIG. 1, the contents of which are further described in this example.
TABLE 2Average TAF CC10 ConcentrationsCC10CC10CC10CC10 Conc. inConc. inConc. inConc. inTAF**TAFTAFTAF12 Hours24 Hours48 Hours72 HoursPlacebo 476 ng / ml 753 ng / ml 916 ng / ml3435 ng / ml1.5 mg / kg*2336 ng / ml1639 ng / ml2492 ng / ml1522 ng / mlrhCC105 mg / kg*2400 ng / ml1...
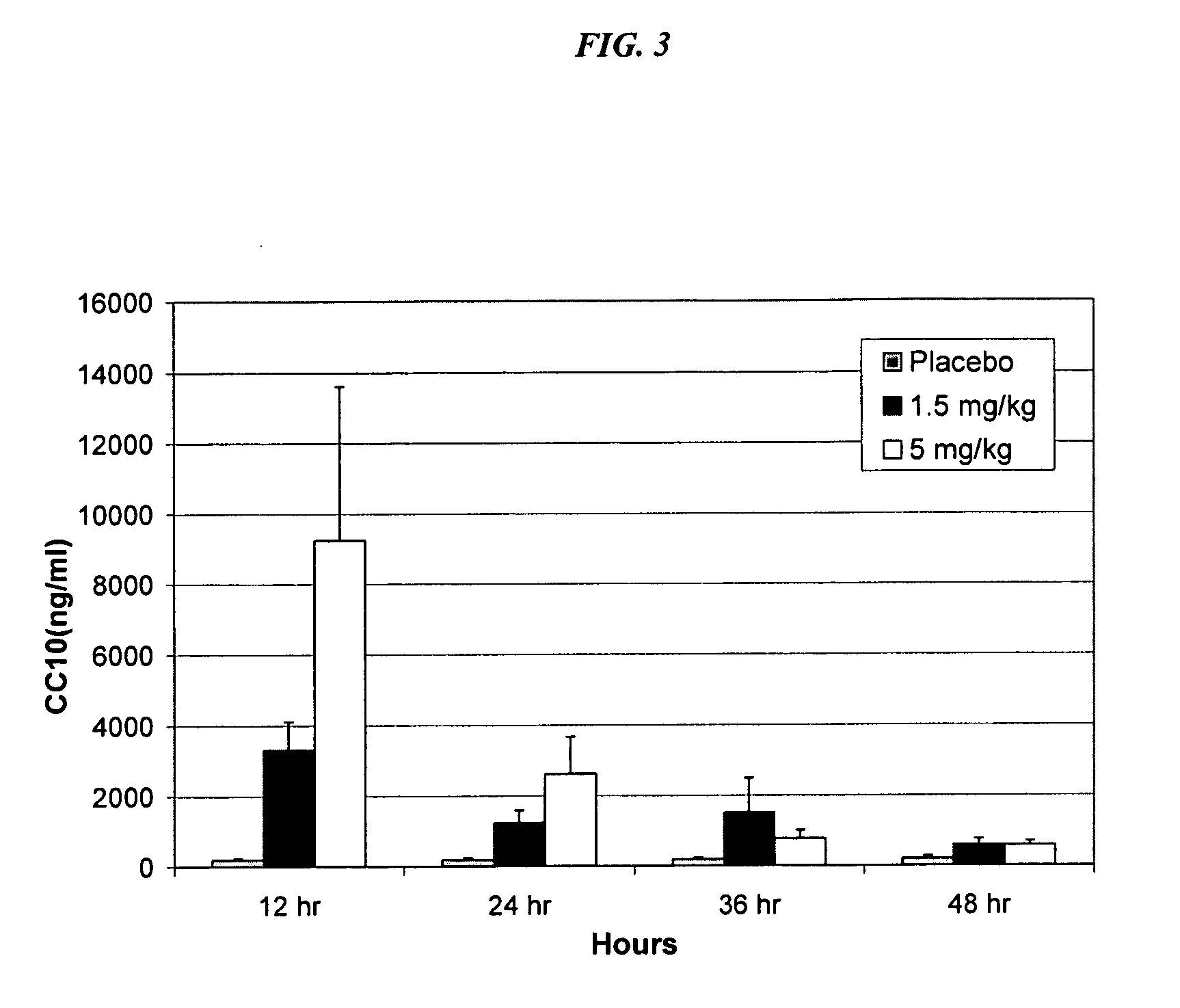
example 3
Serum Concentrations of CC10 in Patients Treated with rhCC10
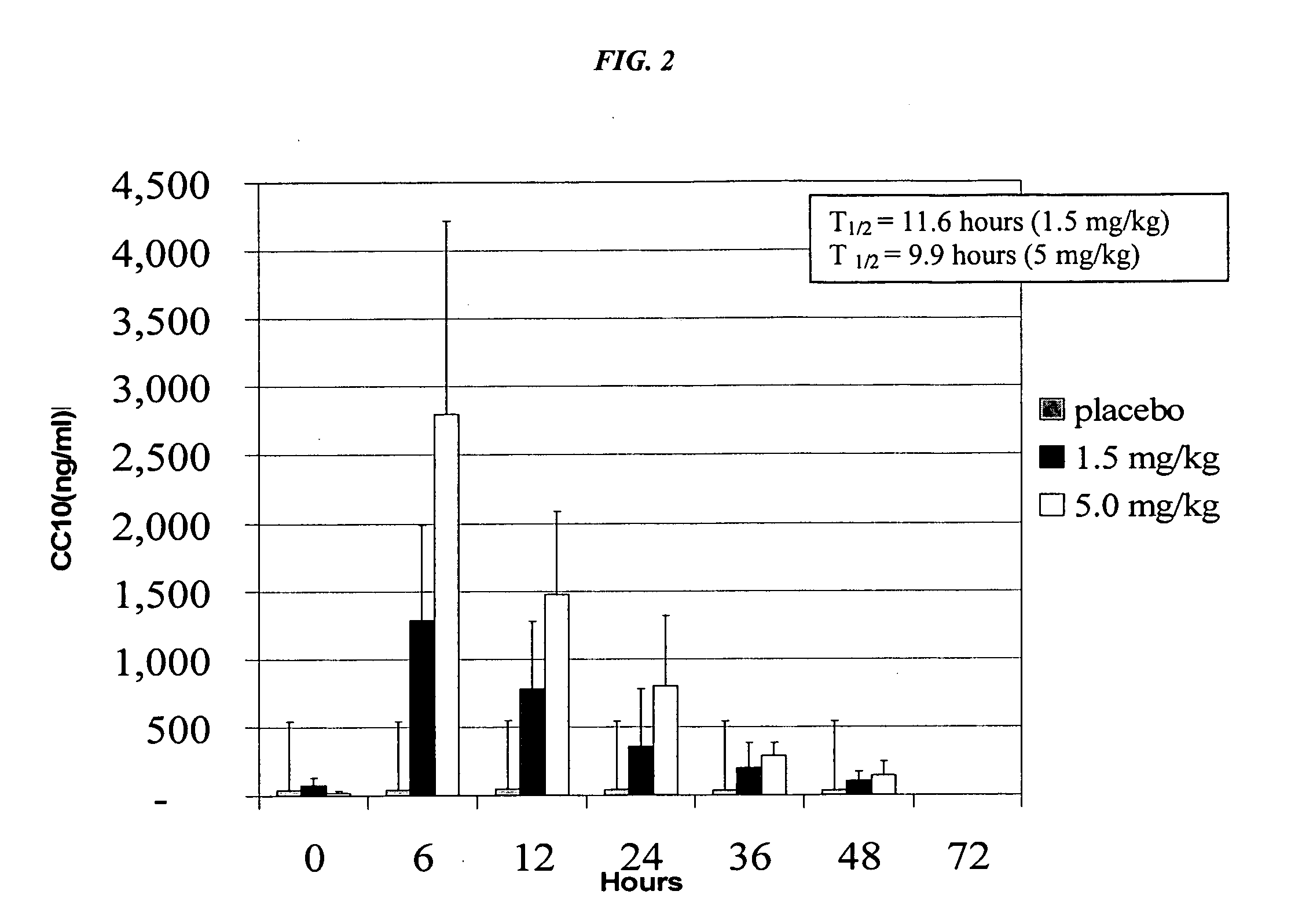
[0064] Furthermore, with reference to FIG. 2, in one embodiment, rhCC10 may be administered intratracheally such that peak serum levels of CC10 are achieved within 6 hours of administration. Peak serum levels occur within 6 hours, irrespective of the dose of rhCC10 administered. Based on the results described below and in FIG. 2, peak serum levels will occur within about 6 hours after administration across all dosage ranges.
[0065] Reference is now made to Table 3, as well as to FIG. 2, the contents of which are further described in this example.
TABLE 3Average Serum CC10 ConcentrationsCC10CC10CC10CC10CC10CC10Conc. inConc. inConc. inConc. inConc. inConc. inserum**serum**serum**serum**serum**serum**Elimination0 Hours6 Hours12 Hours24 Hours36 Hours48 HoursHalf-lifePlacebo42 ng / ml 43 ng / ml 46 ng / ml 40 ng / ml 37 ng / ml 38 ng / mlNotapplicable1.5 mg / kg*76 ng / ml1289 ng / ml782 ng / ml354 ng / ml196 ng / ml101 ng / ml11.6 hoursrhCC10 5 mg / kg...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com