Use of indole-derived compounds for the preparation of a medicament that can be used to treat diseases related to the splicing process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
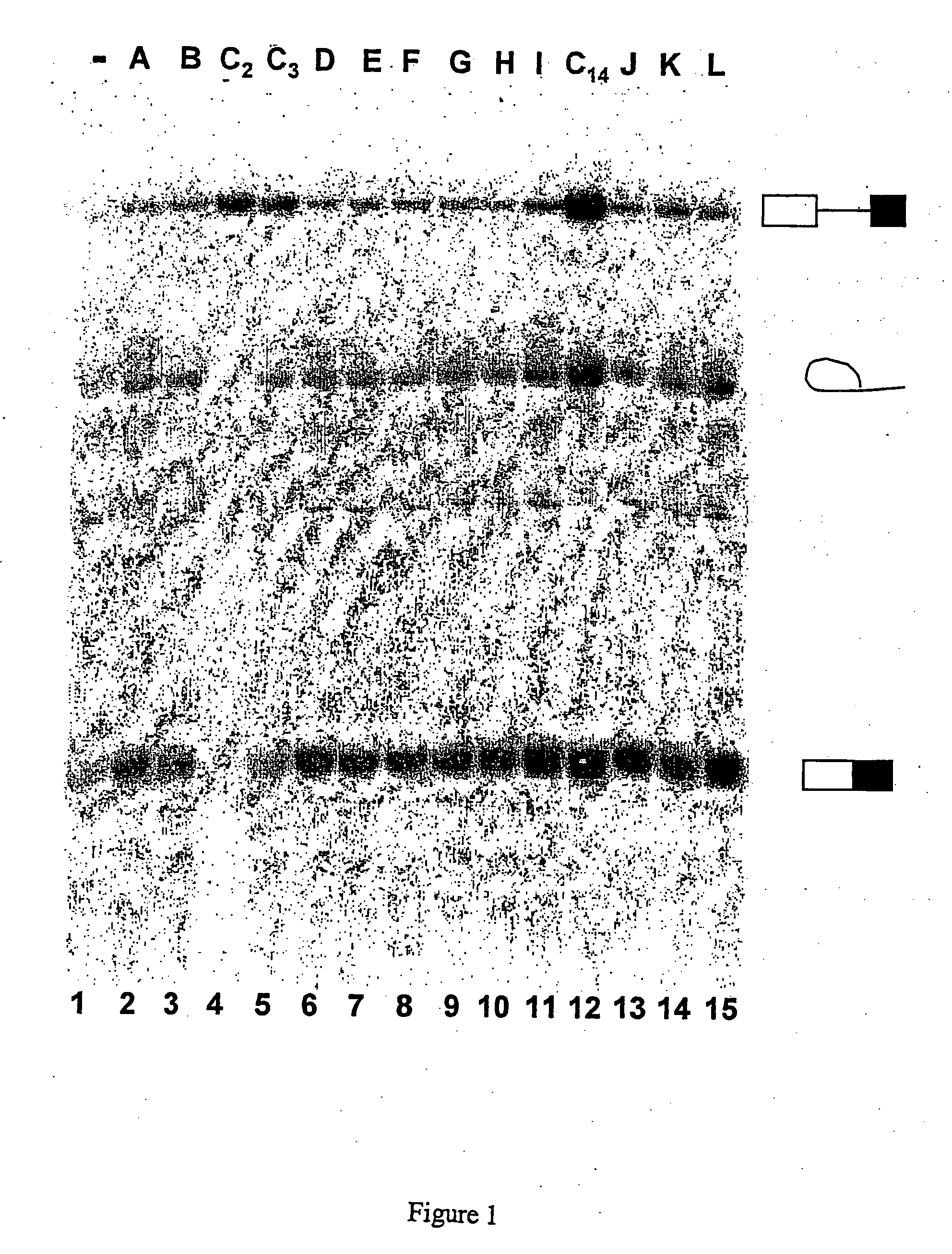
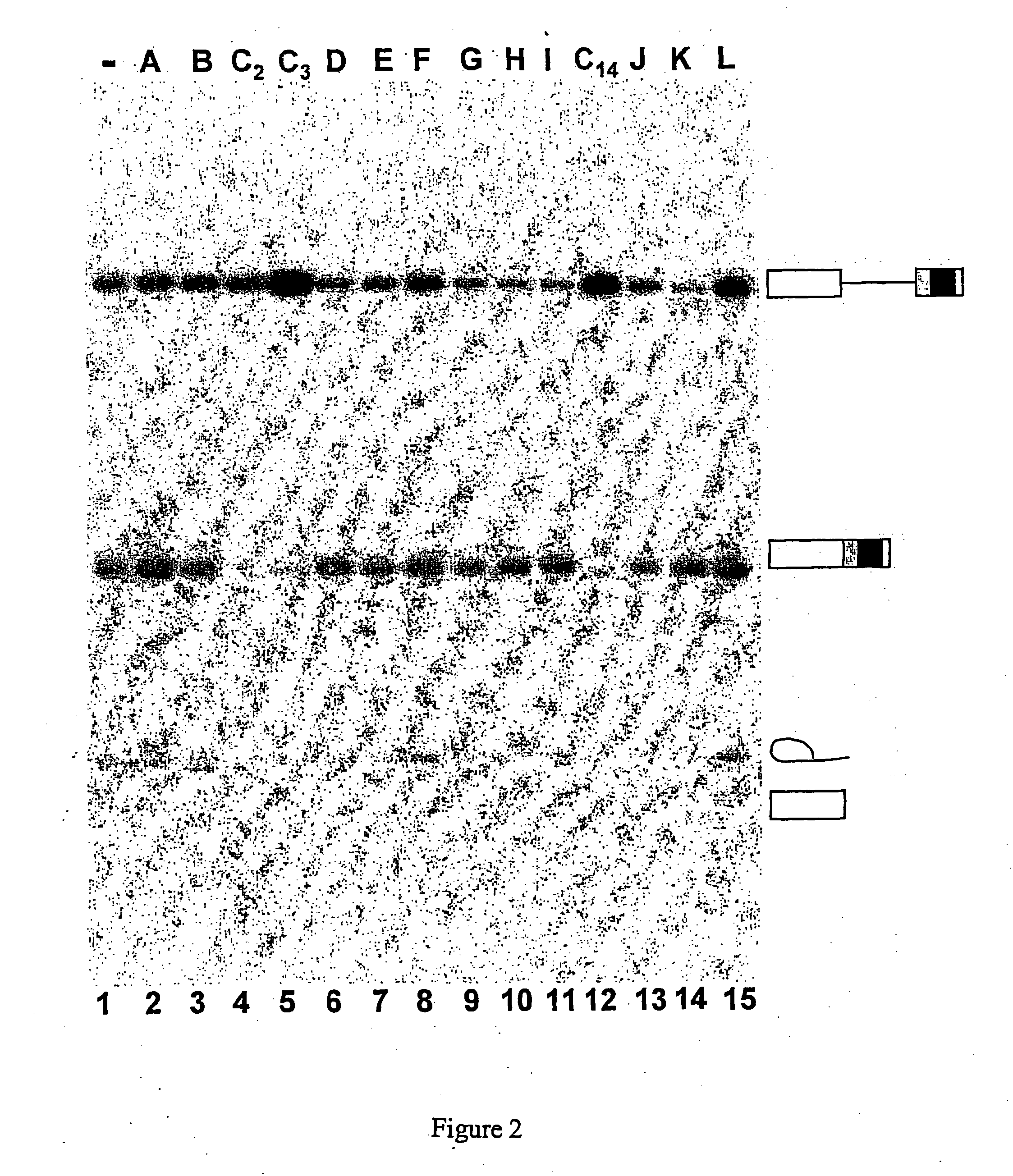
[0300] In vitro inhibition of, splicing of two types of model pre-mRNAs
[0301] The compounds presented in Tables 1 and 2 below were tested in a concentration range of 1 μM, 10 μM and 100 μM, and were selected initially on the basis of their capacity to inhibit, in vitro, the splicing of two types of model pre-mRNAs.
TABLE 1MoleculecodeChemical formulaNomenclatureC1N′-(9-methoxy-5,6,11- trimetyl-6H-pyrido[4,3- b]carbazol-1-yl)-N,N-dimethyl- propane-1,3-diamineC2N′-(9-methoxy-6,11-dimethyl- 6H-pyrido[4,3-b]carbazol-1- yl)-N,N-dimethyl-propane-1,3- diamineC310-chloro-2,6-dimethyl-2H- pyrido[3′,4′:4,5]pyrrolo[2,3- g]isoquinolineC49-hydroxy-5-methyl-6H- pyrido[4,3-b]carbazol-1-yl acetic acid esterC51-(3-dimethylamino- propylamino)5-methyl-6H- pyrido[4,3-b]carbazol-9-olC69-methoxy-5-methyl-6H- pyrido[4,3-b]carbazol-1- carbaldehyde oximeC7N′(9-methoxy-5-methyl-6H- pyrido[4,3-b]carbazol-11-yl)- N,N-dimethyl-propane-1,3- diamineC8N,N-diethyl-N′-(9-methoxy-5- methyl-6H-pyrido[4,3- b]carbazol...
example 2
[0314] In vivo inhibition of the ESE-dependent splicing of GFP (green fluorescent protein) mRNA
[0315] In order to test the efficiency of indole derivatives ex vivo, fibroblast HeLa cell lines were established stably expressing a transgene corresponding to GFP whose sequence was interrupted by an ESE sequence flanked by two identical introns of the human beta-globulin gene described in Example 1 (see FIG. 5A).
[0316] To detect the messenger RNAs arising from, the splicing of this gene, the RT-PCR technique was used with primers in the GFP sequence on each side of the ESE and the PCR products were analyzed on agarose gel.
[0317] In almost all the lines established, a single fragment of 250 base pairs (bp) was amplified by PCR (FIG. 5A, lanes 2 and 3) and it corresponds to an RNA messenger which included the ESE between the two GFP sequences.
[0318] The result indicates that the ESE has a dominant effect and that the RNA messenger produced after splicing contains the two parts of the ...
example 3
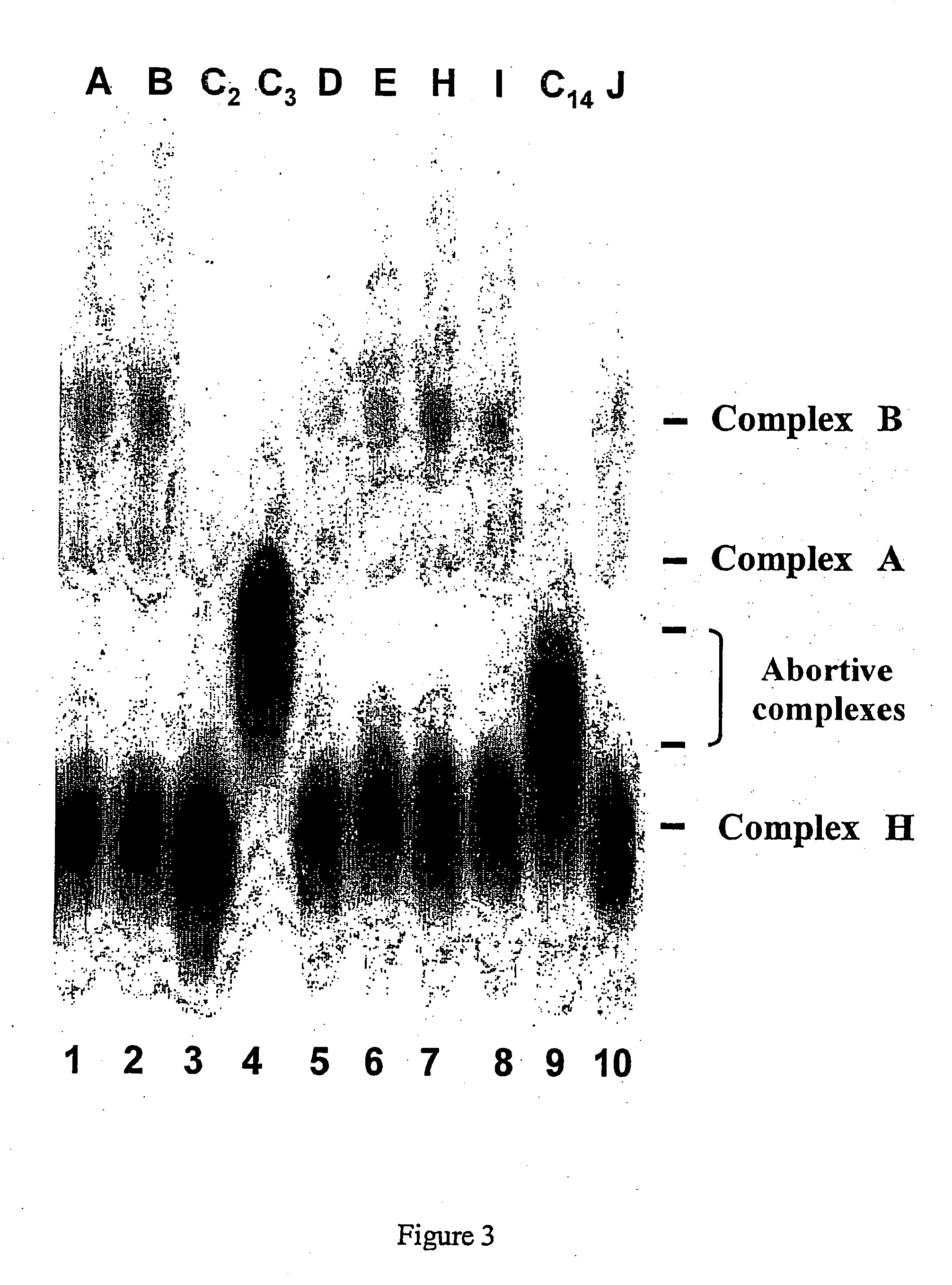
[0322] In vivo inhibition of the ESE-dependent splicing of pyruvate dehydrogenase E1α subunit mRNA
[0323] In order to demonstrate the selectivity of the action on ESE sequences by the compounds according to the invention, it was decided to use another model substrate containing two introns whose splicing depends on two different ESE sequences. In this substrate the sequences corresponding to exon 7-intron 7-exon 8 of the gene coding for the pyruvate dehydrogenase E1α subunit (PDH E1α) are inserted downstream from the M3S1 sequence (M3S1-PDH, see FIG. 4A). Intron 7 of this transcript contains a point mutation which creates a high affinity SR hSC35 protein binding site. This mutation, which causes the loss of PDH E1α expression in patients suffering from Leigh syndrome (an encephalopathy in children), leads to the appearance of a cryptic site 46 nucleotides downstream from the authentic 5′ splicing site (FIG. 4A). This substrate, likely to give rise to two products, one with and one w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com