Oxygen delivery agents and uses for the same
a technology of oxygen delivery agent and oxygen, applied in the field of oxygen delivery agent or blood substitute, can solve the problems of limited thermal expansion ability and practical constraints
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Gas Filled Vesicles
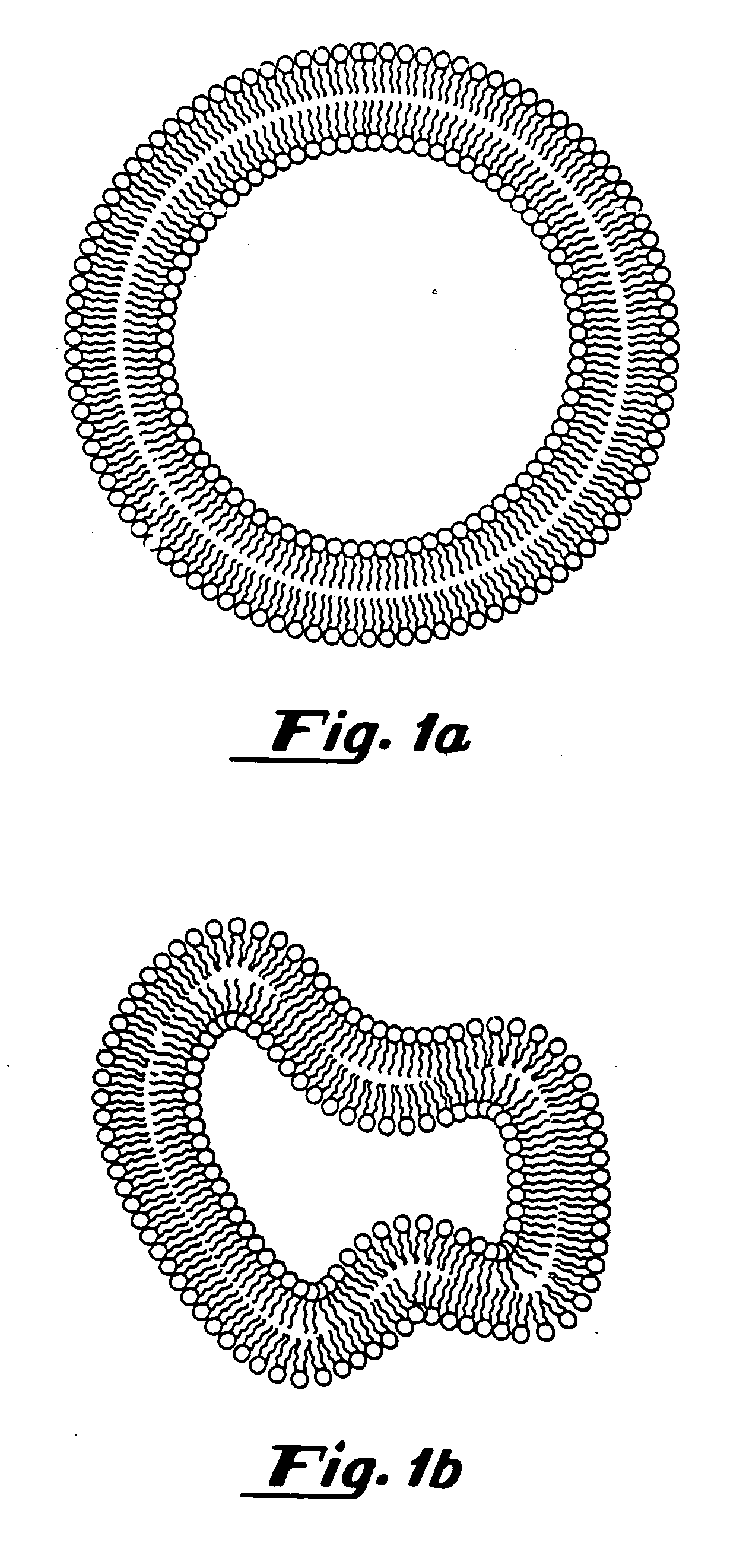
[0285] To prepare gas filled vesicles, a 5 ml solution of a lipid mixture (5 mg / ml) containing 87 mole % dipalmitoylphosphatidylcholine (DPPC), 8 mole % dipalmitoyl-phosphatidylethanolamine-polyethylene glycol-5000 (DPPE-PEG-5000), 5 mole % dipalmitoylphosphatidic acid (DPPA) (all supplied by Avanti Polar Lipids, Alabaster, Ala.) in 8:1:1:1 normal saline:glycerol:propylene glycol solution, was placed in a 15.8 ml glass bottle with a rubber stopper. Air was evacuated from the bottle using a vacuum pump (Model Welch 2-Stage DirecTorr Pump, VWR Scientific, Cerritos, Calif.) by connecting the hose to the bottle through an 18 gauge needle perforating the rubber stopper. After removing the air, perfluorobutane (Pfaltz and Bauer, Waterbury, Conn.) was placed in the stoppered bottle via another 18 gauge needle connected to tubing attached to a canister of perfluorobutane. This process was repeated 5 times so that any traces of air were removed from the sto...
example 2
Methods of Sizing Gas Filled Vesicles
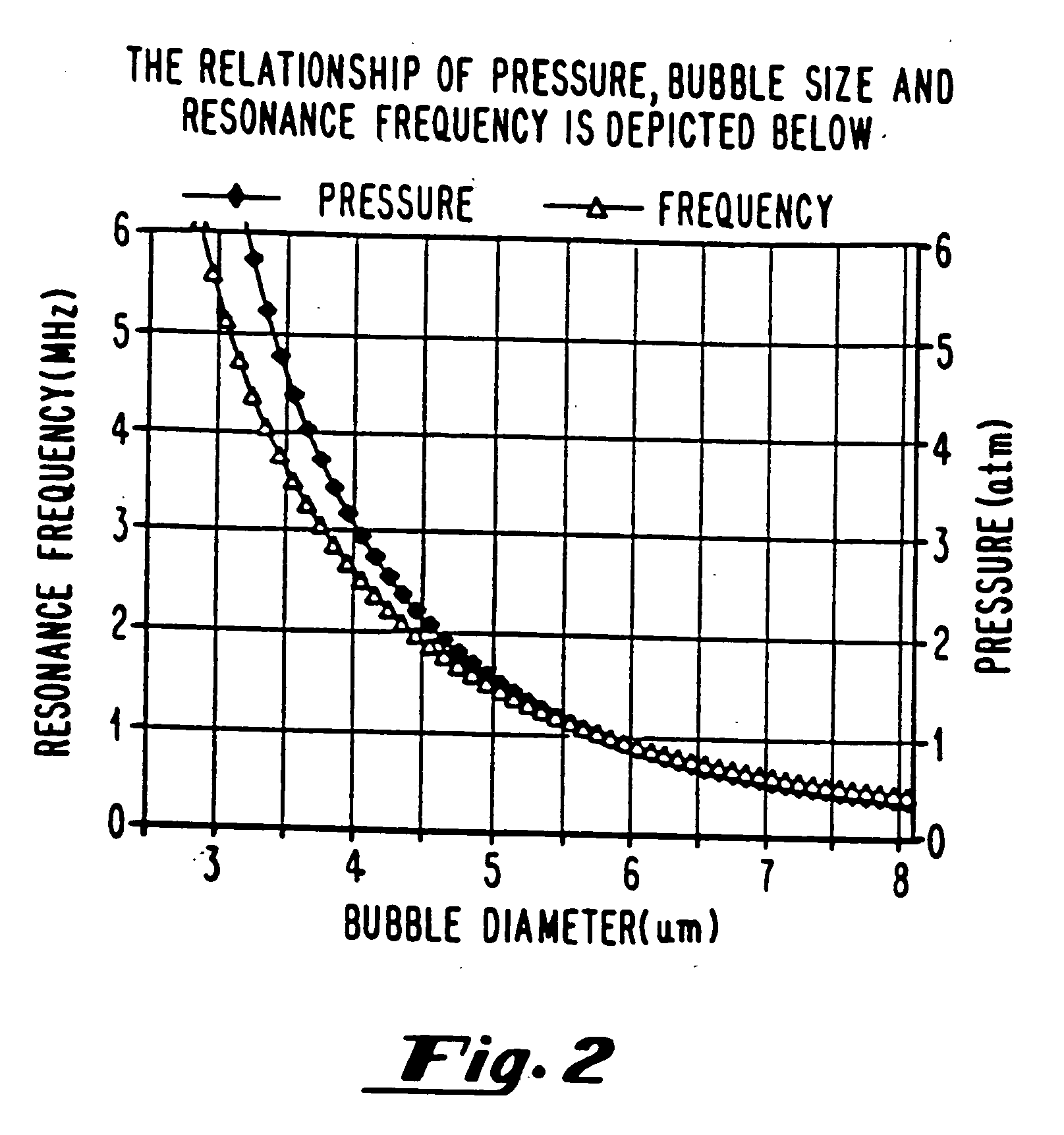
[0288] The first method for sizing gas filled vesicles is applicable with diluents with different gas compositions, for example air-saturated saline, nitrogen-saturated saline or degassed saline. A mechanical diffusion pump is throttled to achieve various gas concentrations in the solutions. The level of oxygen is detected with an oxygen sensitive electrode. The “collect data” feature on an optical particle sizer is used with dilute solutions, otherwise the “autodilute” function is used. The accuracy of the particle count is a function of concentration and is more accurate for dilute solutions.
[0289] The second method for sizing gas filled microspheres is optical microscopy, which employs an analytical program for averaging several frames of sampling. Size distribution, concentration and statistical analysis of variation can all be determined this way.
[0290] A 200 ml lipid solution (i.e., 200 mg of the lipid mixture described in Example 1 in 2...
example 3
In Vivo Admimistration of Gas Filled Vesicles
[0291] Lipid vesicles were prepared following the method in Example 1, except that the gas used was pertluoropropane or a combination of 80% perfluoropropane and 20% air. As would be known to the skilled artisan, air comprises about 21% oxygen, so that 20% air would comprise approximately 4% oxygen.
[0292] Ten groups of mice were injected with the lipid vesicles (e.g., the lipid mixture from Example 1)containing perfluoropropane. For Groups 1-5, the lipid vesicles contained 100% perfluoropropane. For Groups 6-10, the lipid vesicles contained 80% perfluoropropand and 20% air.
[0293] The deaths of the mice exposed to the lipid vesicles in Groups 1-10 were recorded after one hour. In Groups 1-5, which were exposed to lipid vesicles containing 100% perfluoropropane, only mice receiving over 4.0 cc / kg body weight died. The actual mortality rate was 60% in mice receiving this dose. On the other hand, in Groups 6-10, which were exposed to lipid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com