Interleukin-12 as an adjuvant for paramyxoviridae vaccines
a vaccine and interleukin-12 technology, applied in the field of interleukin-12 as an adjuvant for paramyxoviridae vaccines, can solve the problems of increased hospitalization rate, rsv-sensitive mutants, and vaccine elicited complement-binding antibodies but failed to protect against infection in children, so as to reduce the replication rate of respiratory syncytial virus (rsv), the effect of potent adjuvant effect and reducing viral replication
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Immunization of Mice with RSV and IL-12
[0033] Mice: Pathogen-free female BALB / c mice, 8 to 10 months old, were purchased from Charles River Laboratories (Raleigh, N.C.) and cared for according to the “Guide for the Care and Use of Laboratory Animals” as previously described (Graham, B. S., et al, J. Med. Virol. 26:153 (1988)).
[0034] RSV Immunogen and Virus: Preparation of the formalin-inactivated alum-precipitated RSV and preparation of stock of the A2 strain of RSV have been previously reported (Graham, B. S., et al, Immunol. 151:2032 (1993)). Both the vaccine preparation and the challenge stock were derived from the A2 strain of RSV.
[0035] Murine Cytokine IL-12: Murine recombinant IL-12 was expressed from cloned cDNAs (Schoenhaut, D. S., et al, J. Immunol. 148:3433 (1992)). The lot used in this paper was MRB021693-1.2 (Genetics Institute, Cambridge, Mass.) with a specific activity of 5.6×106 units / mg as determined by PHA blast assay (Wolf, S. F., et al, J. Exp. Med. 146:3074 (1...
example 2
Effect of Delivery Route of IL-12 on its Adjuvant Ability
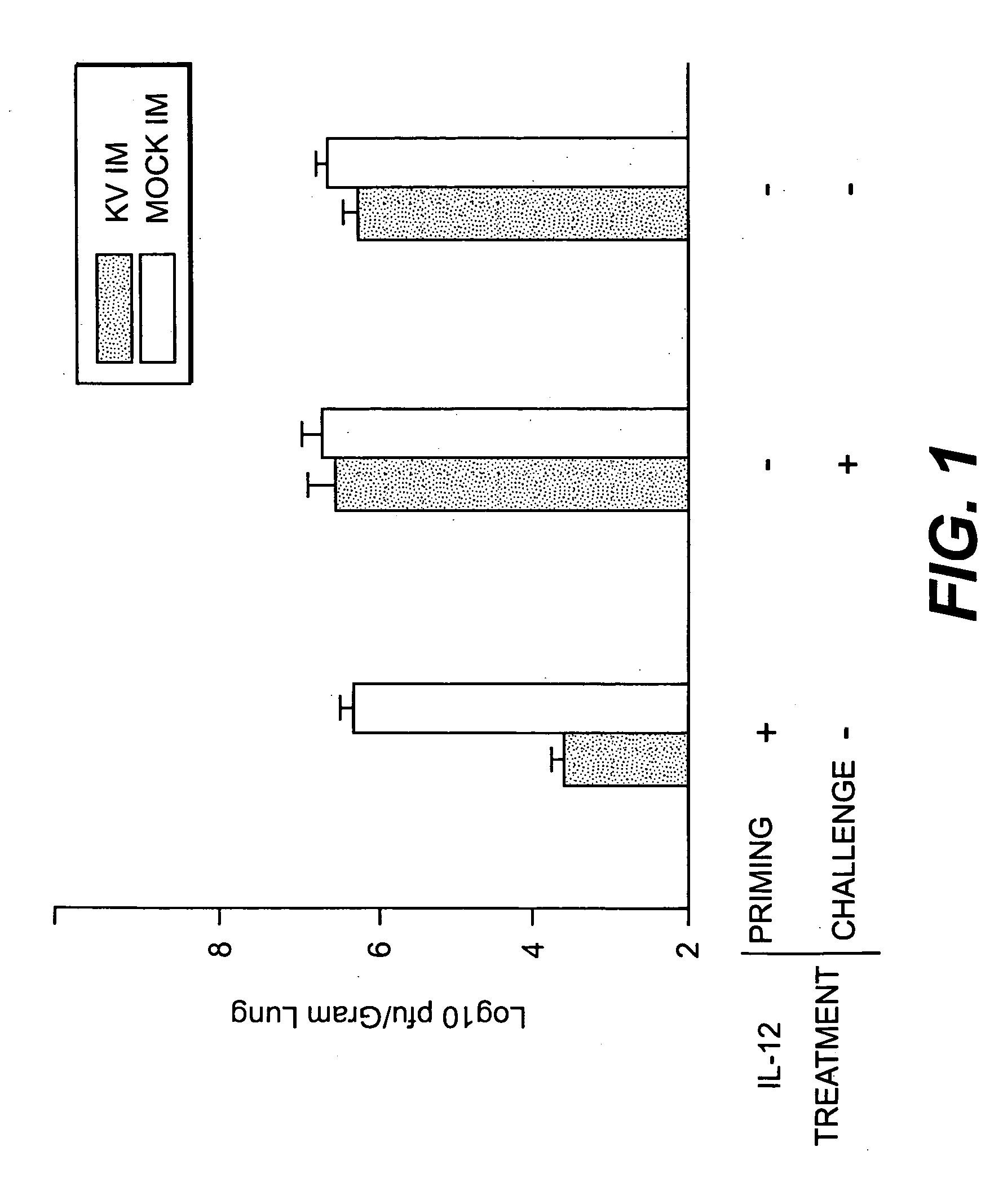
[0040] The effect of a different delivery route of the adjuvant IL-12 was assessed. IL-12 was administered intraperitoneally as described in Example 1 to one group of mice. To another group of mice, IL-12 was administered intramuscularly mixed with the RSV antigen. The control mice were either mock immunized or treated with IL-12 alone without antigen. Table 1 summarizes the results of the experiment which show that a single dosage of IL-12 given simultaneously with immunogen had the same effect on the reduction of viral replication compared to the 5-dosage intraperitoneal regimen. IL-12 as a specific immunomodulator only worked in RSV immunized mice, having no effects on the unprimed mice. See FIG. 1 and Table 1. These data demonstrate that IL-12 exerted a potent adjuvant effect on the inactivated RSV immunogen.
example 3
Assay of Immunoglobulin Isotype Titers in RSV-immunized Mice Receiving IL-12
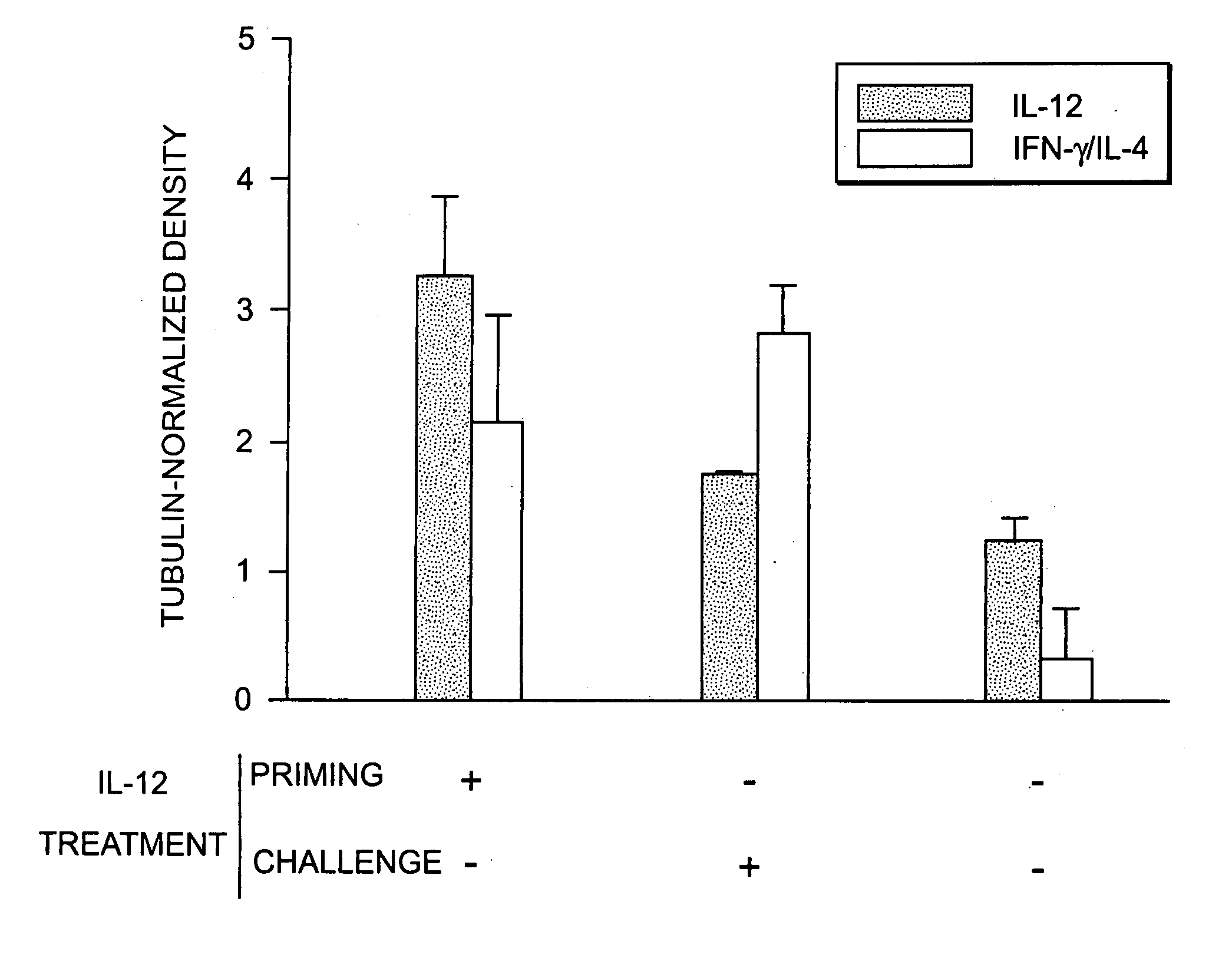
[0041] The patterns of immunoglobulin isotypes produced in RSV-immunized mice receiving IL-12 was examined. Mouse serum samples were collected on the day of and two weeks after live RSV challenge.
[0042] RSV-Specific Immunoglobulin Isotype ELISA: All serologic assays were performed by a person blinded to the experimental groups. BCH4 and BC cells were bound to the solid phase on Immulon II 96-well plates (NUNC, Denmark). Serial diluted mouse serum samples were added to each well. Plates were incubated, washed, and goat anti-murine IgG1 or IgG2a conjugated to alkaline phosphatase (Southern Biotechnology, Birmingham, Ala.) diluted 1:1000 was added, respectively. After another incubation, plates were washed and substrate was added for 30 minutes at room temperature and OD405 was determined (Graham, B. S., et al, Immunol. 151:2032 (1993); Tang, Y.-W., et al, J. Clin. Invest. (1994)). A serum dilution was consid...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| mRNA Northern blots | aaaaa | aaaaa |
| nucleic acid | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com