Methods and compositions for controlling biofilm development
a technology of compositions and biofilms, applied in the direction of biocide, detergent compounding agents, cleaning compositions, etc., can solve the problems of reducing the life of materials and general harsh treatment to both the plumbing system and the environment, so as to improve the life of materials, inhibit, detach, clean, and/or disperse the effect of biofilms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
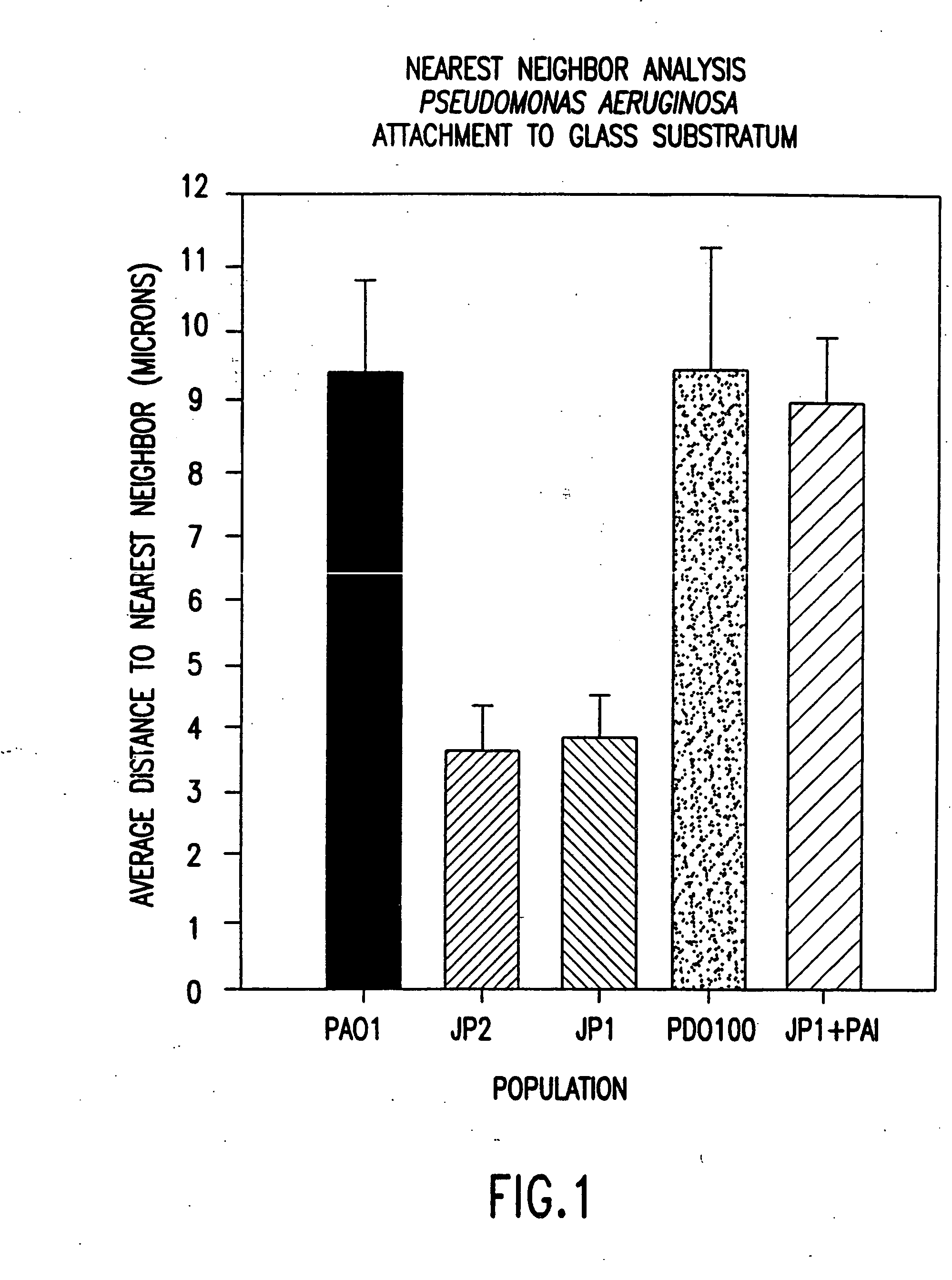
[0106] The most unequivocal experimental design, to determine the role of HSL signal molecules on the formation of biofilms by cells of P. aeruginosa, was to use direct microscopic methods to monitor biofilm formation by cells of HSL negative mutants. For this reason planktonic cells of a wild-type strain (PAO1), and of three mutants incapable of synthesizing specific HSLs, were introduced into flow cells, and adhesion and biofilm formation were monitored by means of confocal scanning laser microscopy (CSLM). Using these techniques, it is possible to monitor the development of live biofilms of the strains of interest.
[0107] Bacteria and media. The Bacteria strains used in this study are listed in Table 1. All experiments were performed using a defined culture medium containing the following, in grams per liter: sodium lactate, 0.05; sodium succinate, 0.05; ammonium nitrate, 0.05; KH2PO4, 0.19; K2HOP4, 0.63; Hutner Salts (Cohen-Bazire, 1957), 0.01; glucose, 1.0; and L-histidine, 0.0...
example 2
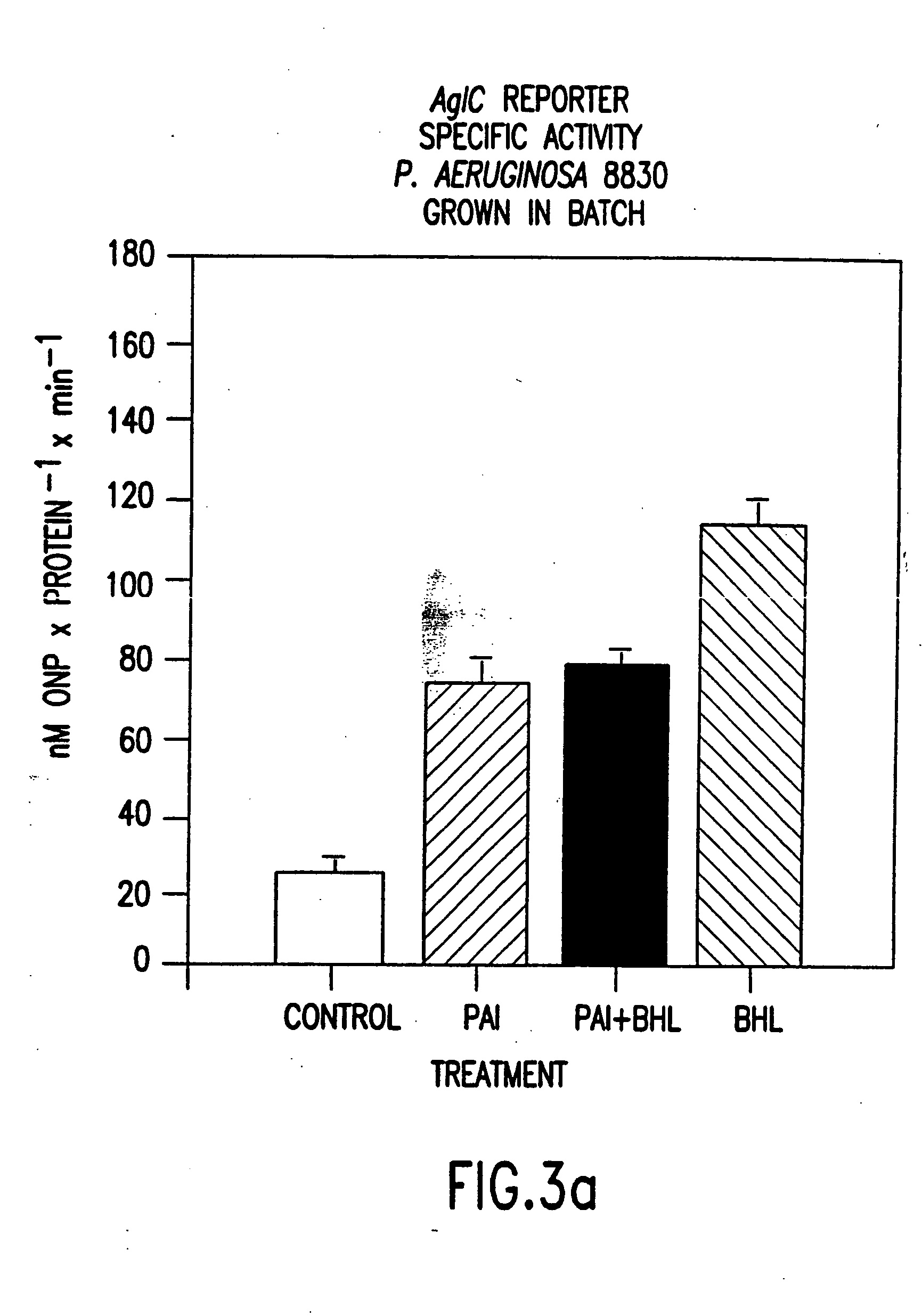
[0114] To confirm that HSL was responsible for the architectural differences noted between wild-type and mutant biofilms, an experiment was performed to demonstrate that addition of filterable material collected from medium in which the wild-type organism had grown would recover the wild-type architecture in the double mutant, P. aeruginosa PAO-JP2. When the double mutant was thus grown as a biofilm, it developed an intermediate from between the wild-type and the untreated double mutant. The interior of the cell clusters appeared similar to the untreated P. aeruginosa PAO-JP2 and the exterior of the cell clusters appeared similar to the wild-type organism.
[0115] This experiment was repeated, culturing the double mutant using a concentration of 10 μM OdDHL in fresh medium. This resulted in recovery of the intermediary phenotype as was observed when the cells were grown in the presence of spent medium. These results indicated that biofilm architecture P. aeruqinosa PAO1 biofilms is c...
example 3
Use of OdDHL Analog to Prevent Normal Biofilm Development
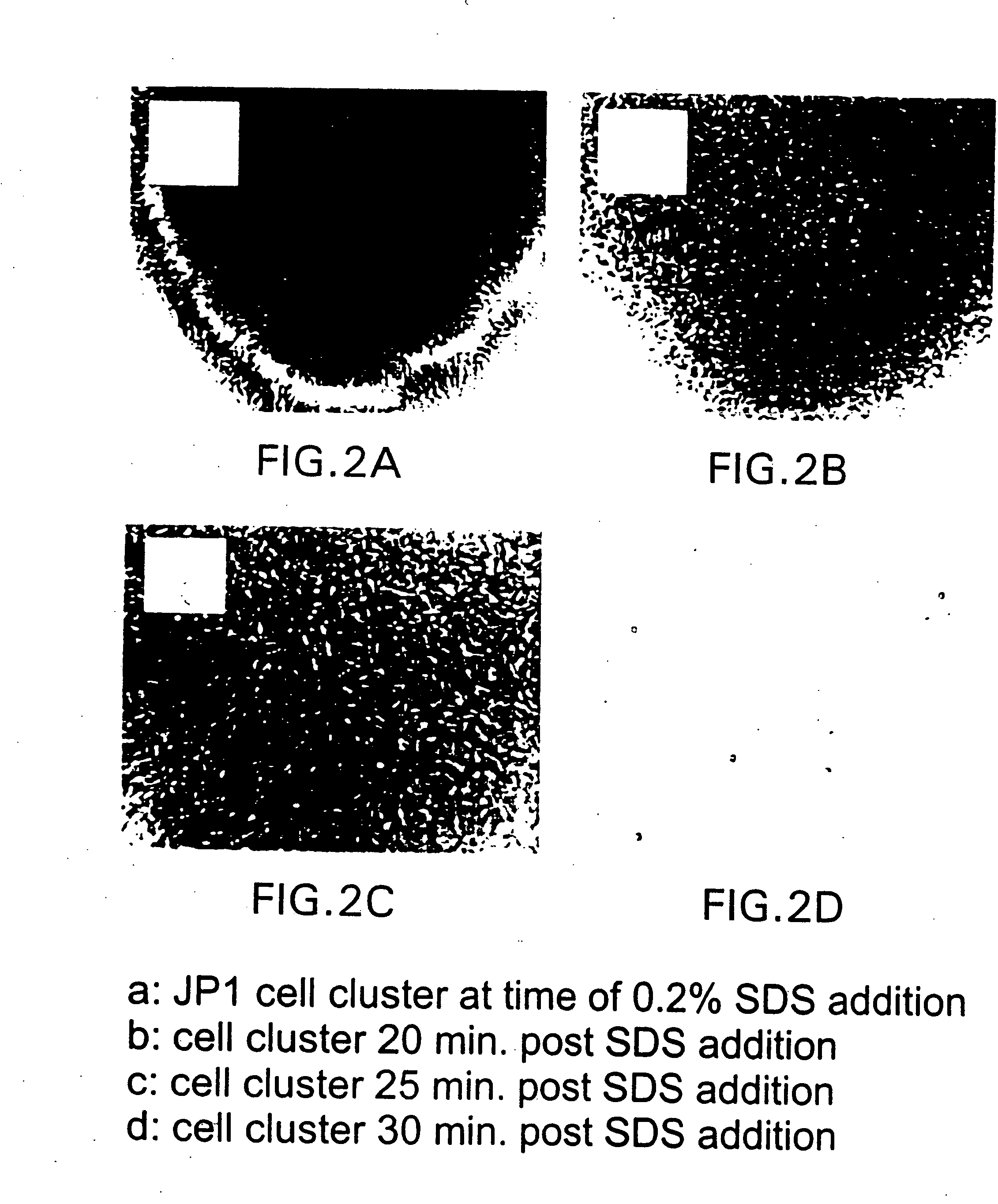
[0122] As a typical embodiment of the invention, an OdDHL blocking agent was used to demonstrate that normal biofilm development could be disrupted and the biofilm dispersed following the addition of 0.2% SDS. An analog of OdDHL, N-(2,2-difluorodecanoyl)-L-homoserine lactone was dissolved in 25% ethanol, 75% water by volume and added at a final concentration of 10 uM to growing biofilm cultures of P. aeruginosa PAO1 wild-type. Following 10 days incubation, the biofilm was analyzed for cell to cell distances, cell cluster thickness and response to treatment with 0.2% SDS. It was shown after 10 days incubation that the average cell to cell distance was less than 4 um and was not significantly different from that observed with the OdDHL knockout mutant P. aeruginosa PAO-JP1. The cell cluster average thickness was under 20 um and was not significantly different from the OdDHL knockout P. aeruginosa PAO-JP1. Finally, when treated ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| average cell cluster depth | aaaaa | aaaaa |
| average cell cluster depth | aaaaa | aaaaa |
| depth | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com