Devices and methods for reducing scar tissue formation
a scar tissue and device technology, applied in the field of scar tissue reduction devices and methods, can solve the problems of scar tissue formation, adhesion and blood vessel narrowing, scar tissue formation risk, etc., and achieve the effect of reducing cellular proliferation and preventing the formation of excess post-operative scar tissu
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
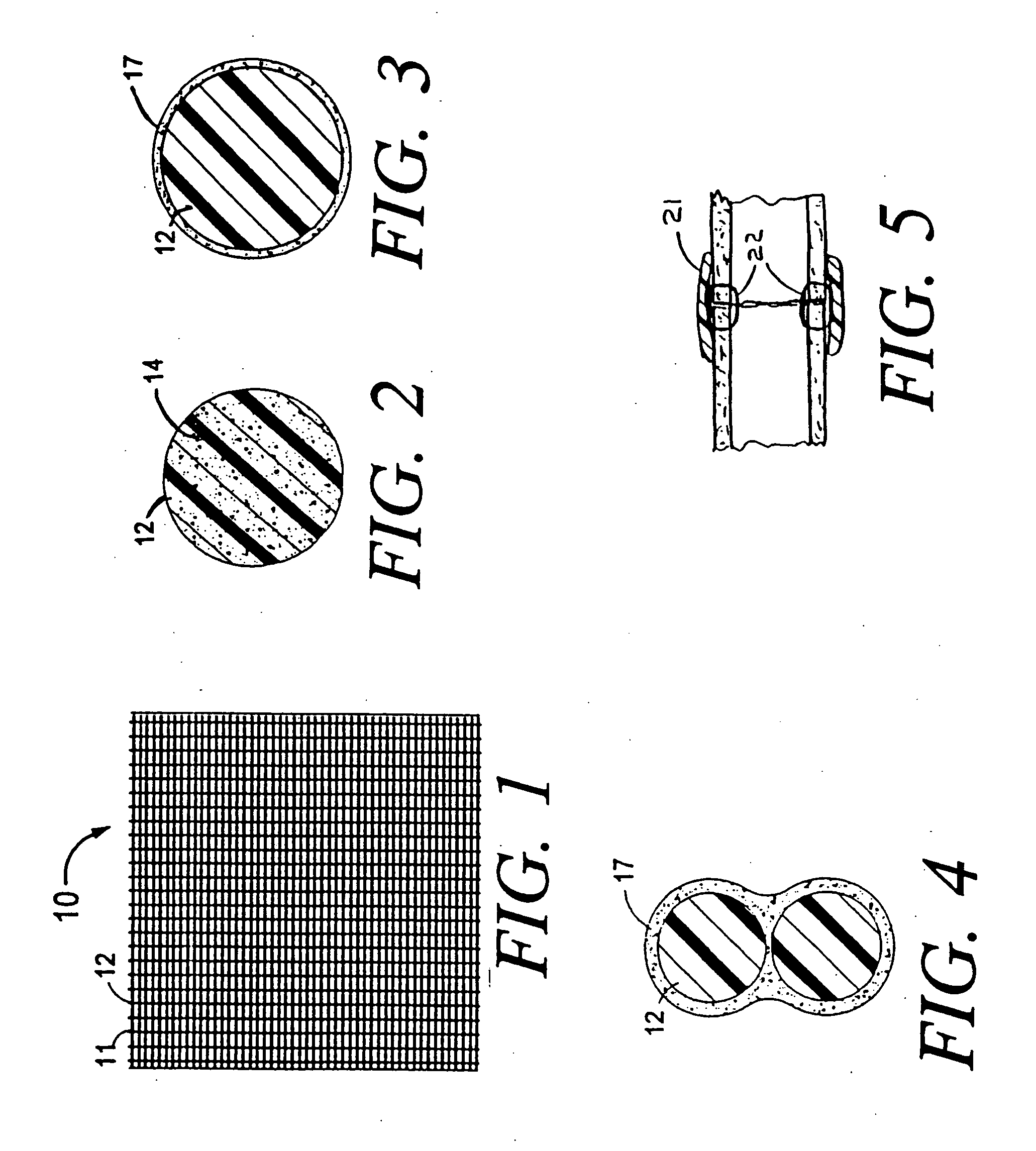
[0030]FIG. 1 shows an absorbable mesh sheet 10 with mesh strands 12 and open spaces 11. The sheet 10 is designed to be placed post-operatively into or around human tissue at the site of a surgical procedure. When placed at the site of a surgical procedure, the sheet 10 is designed to slowly elute a cytostatic drug so as to decrease the formation of scar tissue and to reduce the extent of adhesions. When placed generally around human tissue, the mesh 10 forms a cytostatic anti-proliferative surgical wrap. The mesh strands 12 can be made from oxidized regenerated cellulose or other biodegradable materials with the cytostatic anti-proliferative drug either embedded within the strands, coated onto the outer surfaces of the strands or held onto the strands by adhesion or capillary action. Any of these possibilities will be described herein as the drug being attached to the mesh or attached to the strand of the mesh.
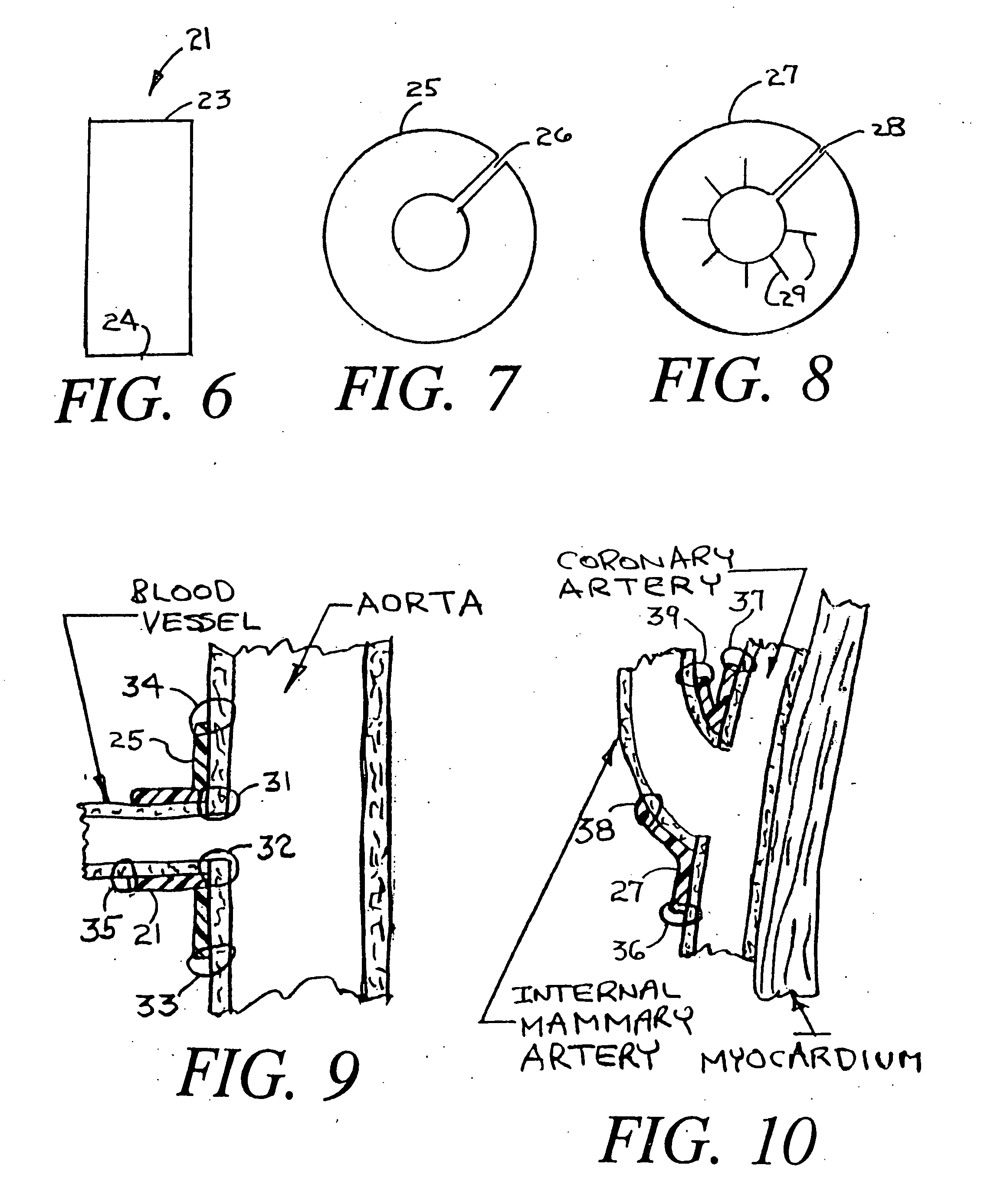
[0031]FIG. 2 is an enlargement of a cross section of a single strand 12 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Biostability | aaaaa | aaaaa |
| Biodegradability | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com