Complex of alpha-fetoprotein and inducers of apoptosis for the treatment of cancer
a technology of alpha-fetoprotein and apoptosis, which is applied in the field of cancer medicine and oncology, can solve the problems of limited afp source, lack of specificity of preparation, and complicated conjugation step of anticancer conjugates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
PAFP Isolation and Purification
[0041] PAFP is collected from the liver and blood of porcine embryos, the amniotic fluid, and the placenta. The time of collection of the fluids is crucial during the pregnancy since it affects the properties of PAFP influencing the parameters and success of the pre-binding step. If collected too early or too late, the glycosylation of PAFP is different and the subsequent binding properties of PAFP are changed. The best time to collect the raw material containing PAFP is between the 6th and 14th week of embryogenesis. The period of collection of the fluids from the embryo is important as the glycosylation of PAFP varies during embryogenesis and the yield of fluids diminishes significantly after the 14th week of gestation.
[0042] The blood and amniotic fluid is kept at 4-10° C. for 12 to 24 hours for natural sedimentation. The supernatant is collected and transferred to a different container. The supernatant is concentrated 3-5 times by ultrafiltratio...
example 2
PAFP is Bioequivalent to HAFP
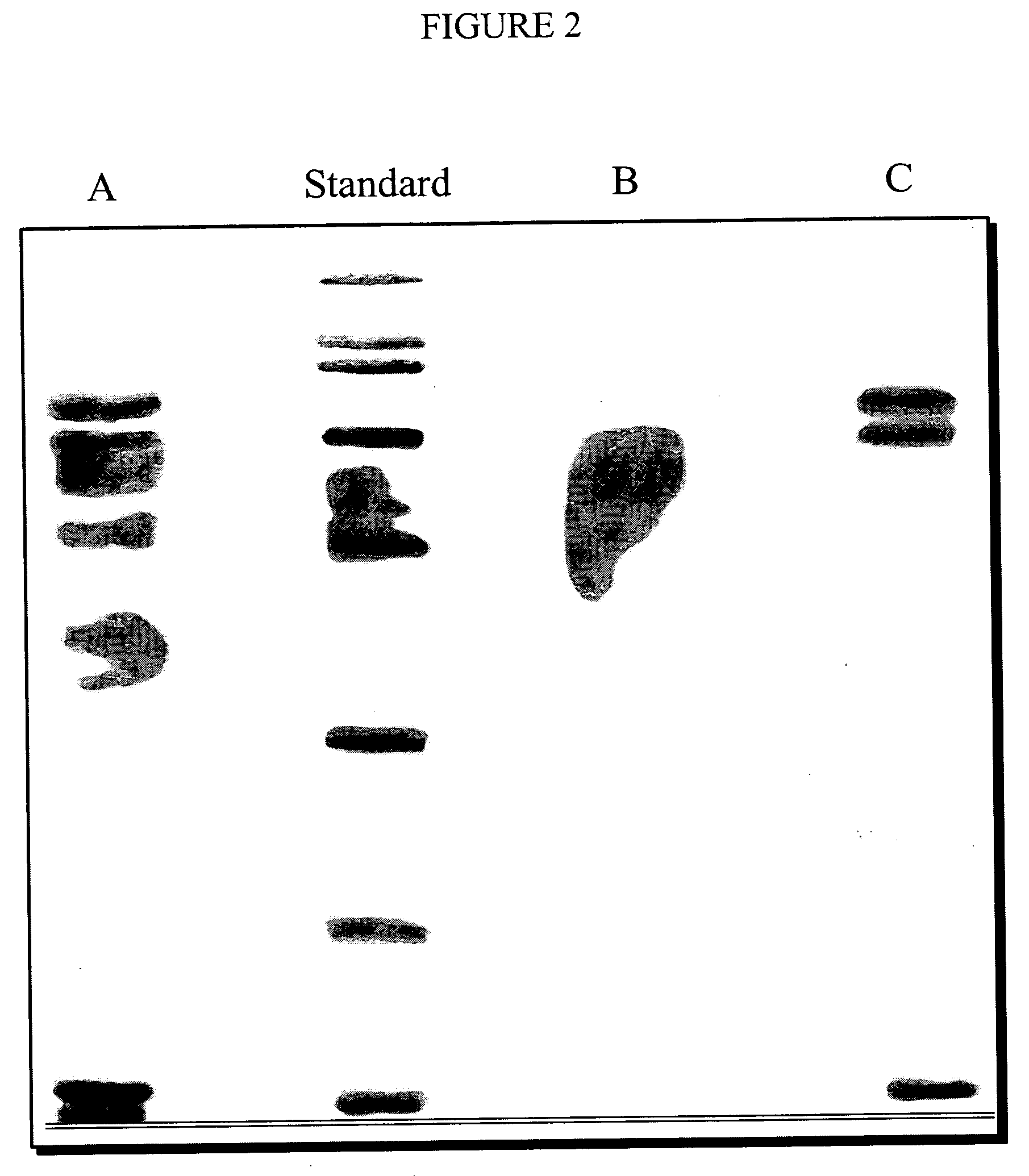
[0044] It was not known if PAFP exhibits the same biological properties as HAFP. Therefore, we tested our preparation of PAFP with 2 immuno enzyme kits for quantitative determination of alpha-fetoprotein in human serum and amniotic fluid. The first one is a membrane EIA Alpha-fetoprotein test (cat. #410-1 produced by IND Diagnostic Inc., Vancouver, Canada) which uses a monoclonal antibody to HAFP. The results were negative confirming a difference of HAFP and PAFP in their chemical structure. The second kit that was used contains a polyclonal antibody to HAFP (T-8456-T-8456-AFP-EIA-BEST-Strip“, manufactured by Vector-BEST, Novosibirsk, Russia) which detected the presence of PAFP. It was not known if PAFP would provoke the same biological response as HAFP (i.e. apoptosis) as the amino-acid ratio for the porcine source is different from the human source. To determine if PAFP is bio-equivalent to the human AFP (HAFP) we: [0045] a. checked if PAFP would bin...
example 3a
In Vitro Binding of PAFP and the First Compound
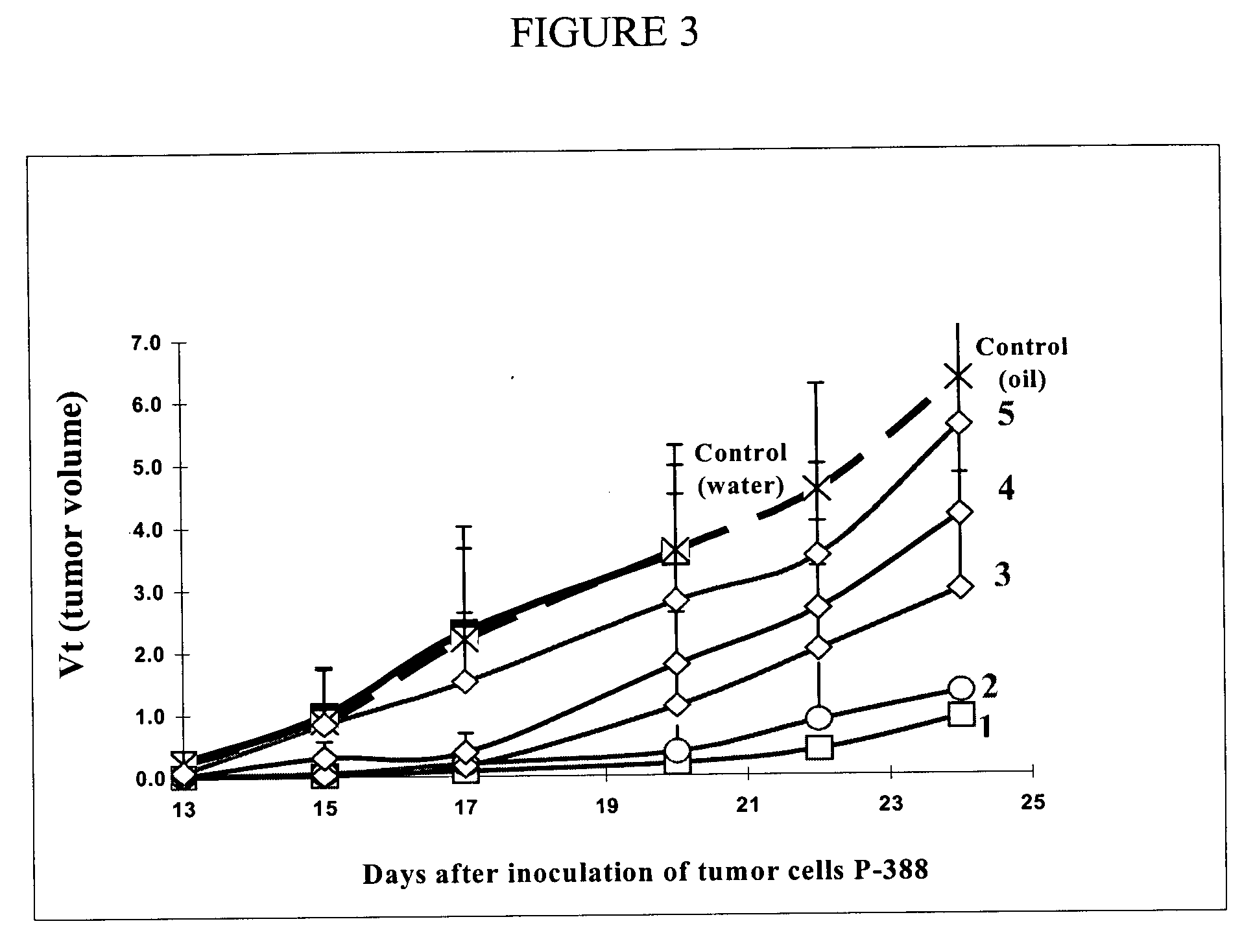
[0047] The upper phase containing unbound PAFP and the first compound (at least one type of anticancer drug) are mixed for one minute and will incubate for an additional 10 minutes at 10-15° C. to allow for the binding of PAFP and the first compound to form a PAFP-first compound mixture. The incubation may occur for a longer time however we find that there is no significant benefit to increasing the time of incubation. The PAFP-first compound mixture then undergoes ultrafiltration using a 50 kDa membrane. We use the diafiltration process to remove small molecules and other non-desired elements (i.e. impurities) such as the salt which is used as a biological buffer during the collection of the raw material (embryo's fluids). We also use the diafiltration step to get rid of any unbound first compound.
[0048] The non-desired elements from the diafiltration are discarded as filtrate and the retentate, containing the PAFP-first compound mi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com