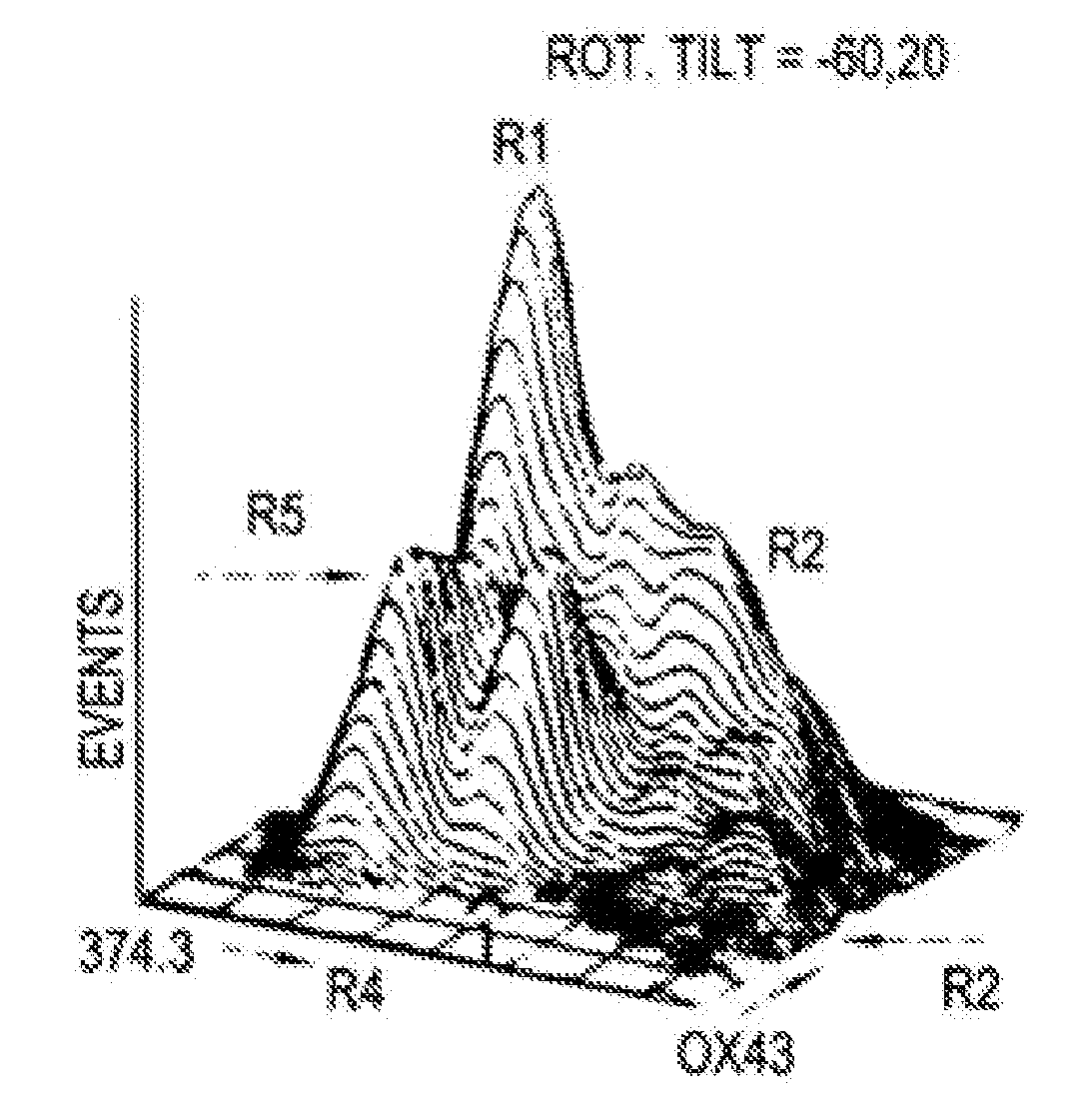
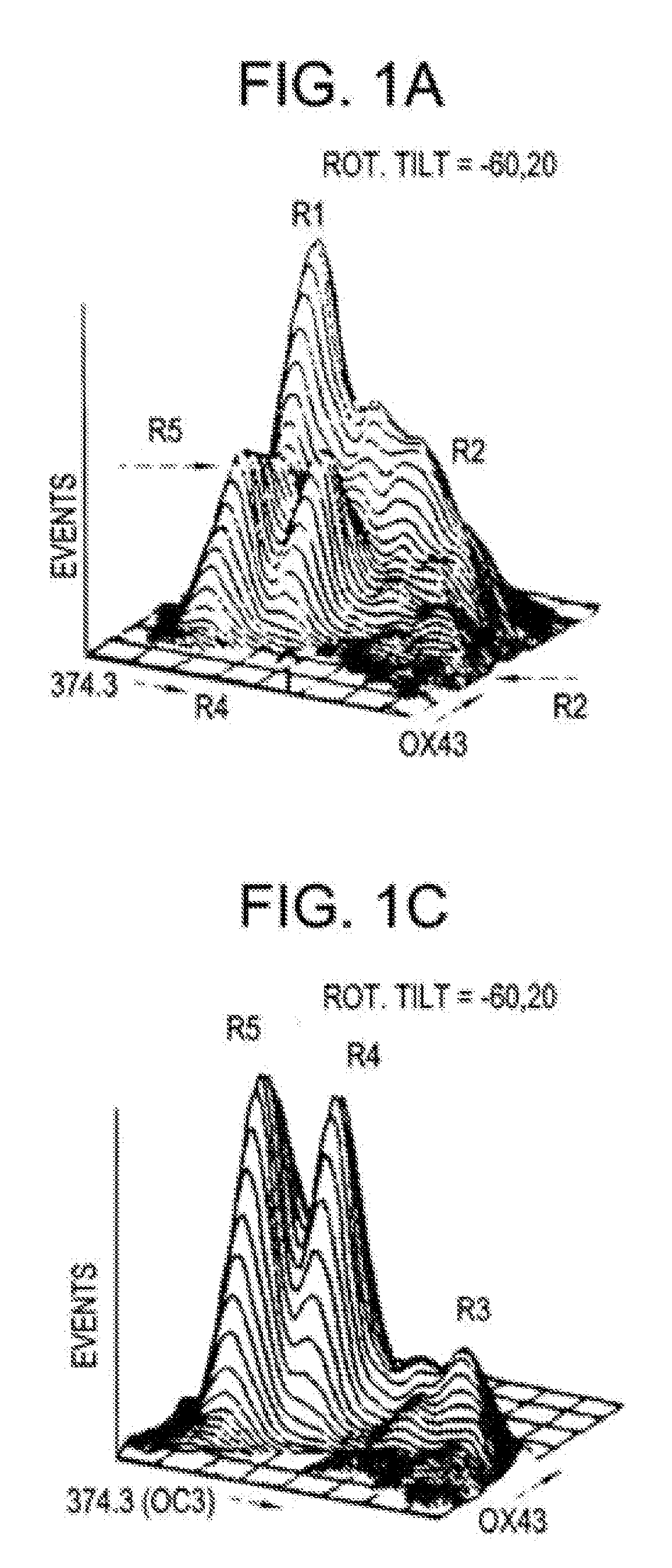
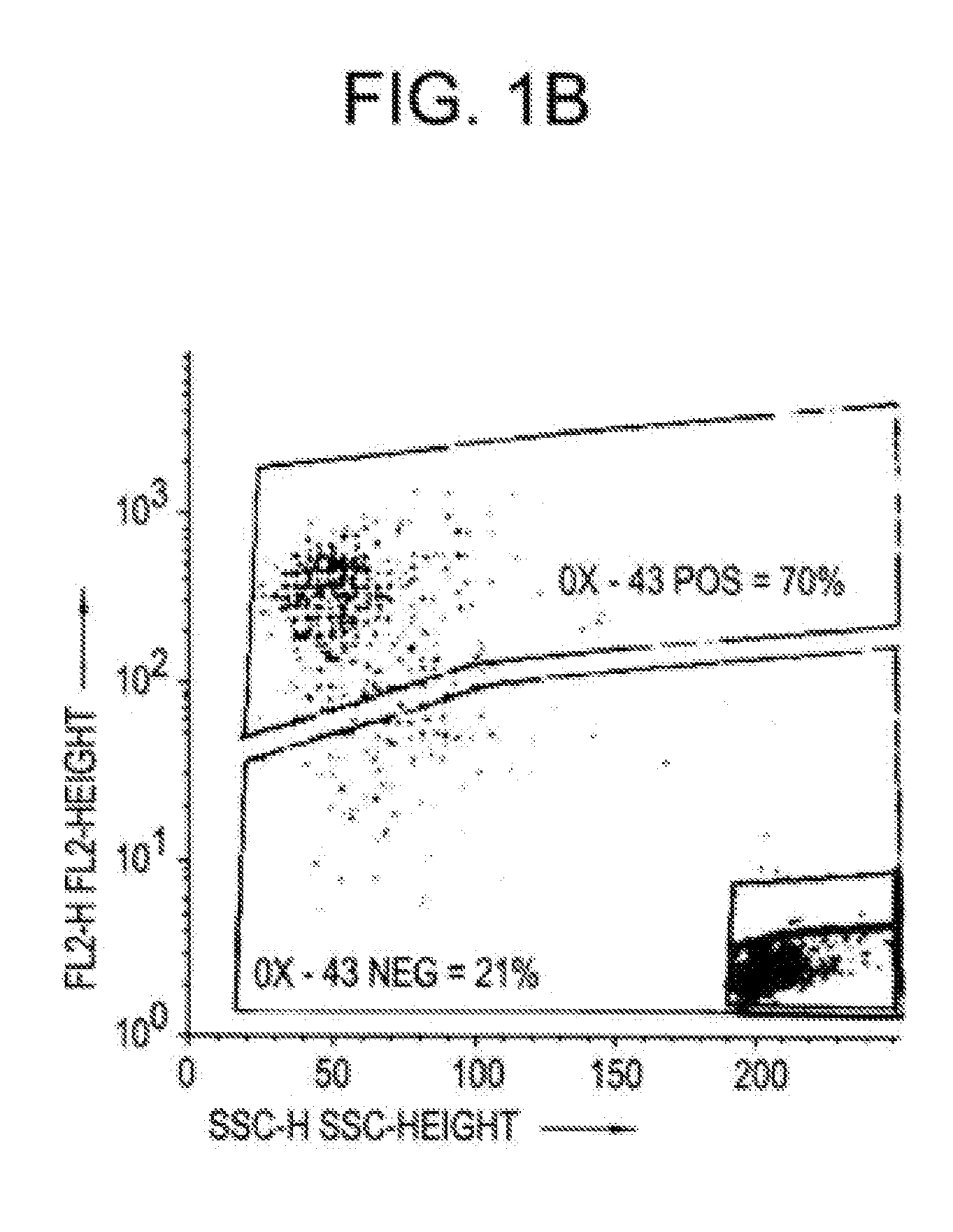
Hepatoblasts and method of isolating same
a technology of hepatoblasts and methods, applied in cell dissociation methods, genetically modified cells, instruments, etc., can solve the problems of difficult isolating of hepatoblasts, limited success in establishing artificial livers with adult liver cells, and difficulty in studying hepatoblasts, so as to reduce the number of contaminating cell types
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example i
[0042] Fischer 344 rats with known durations of pregnancy were obtained from Harlan Sprague Dawley, Inc. (Indianapolis, Ind.) and maintained in the animal facility of the Albert Einstein College of Medicine, Bronx, N.Y. on a standard rat chow diet with 12 hour light cycles. By convention, the first day of gestation is defined as day 0. Use of animals was in accordance with the NIH Policy on the care and use of laboratory animals and was approved by the Animal Care and Use Committee of the Albert Einstein College of Medicine.
[0043] In order to isolate fetal liver cells, pregnant rats at the fourteenth day of gestation were euthanized with ether and the embryos were removed intact and placed into ice cold CA+2-free Hank's Balanced Salt Solution containing 0.04% DNAse, 0.8 mM MgCl2, 20 mM HEPES, pH 7.3 (HBSS). Livers were then dissected from the fetuses and placed into fresh ice-cold HBSS. After all tissues were collected and non-hepatic tissue removed, HBSS-5 mM EGTA was added to a f...
example ii
[0065] Fisher 344 rats with known durations of pregnancy were obtained from Harlan Sprague Dawley, Inc. (Indianapolis, Ind.) and maintained in the animal facility of the Albert Einstein College of Medicine, Bronx, N.Y. on a standard rat chow diet with 12 hour light cycles. By convention, the first day of gestation is defined as day 0. Use of animals was in accordance with the NIH Policy on the care and use of laboratory animals and was approved by the Animal Care and use Committee of the Albert Einstein College of Medicine.
[0066] Pregnant rats at the fifteenth day of gestation were euthanized with ether, and the embryos were delivered. Livers were then dissected from the fetuses, weighed, placed into ice-cold, Ca+2-free Hank's Balanced Saline Solution containing 0.8 mM MgCl2, 20 mM HEPES, pH 7.3 (HBSS), and gently agitated at room temperature for 1 minute. After removal of non-hepatic tissue, livers were gently triturated and then stirred at 37° C. for 10 to 15 minutes in an Erlenm...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com