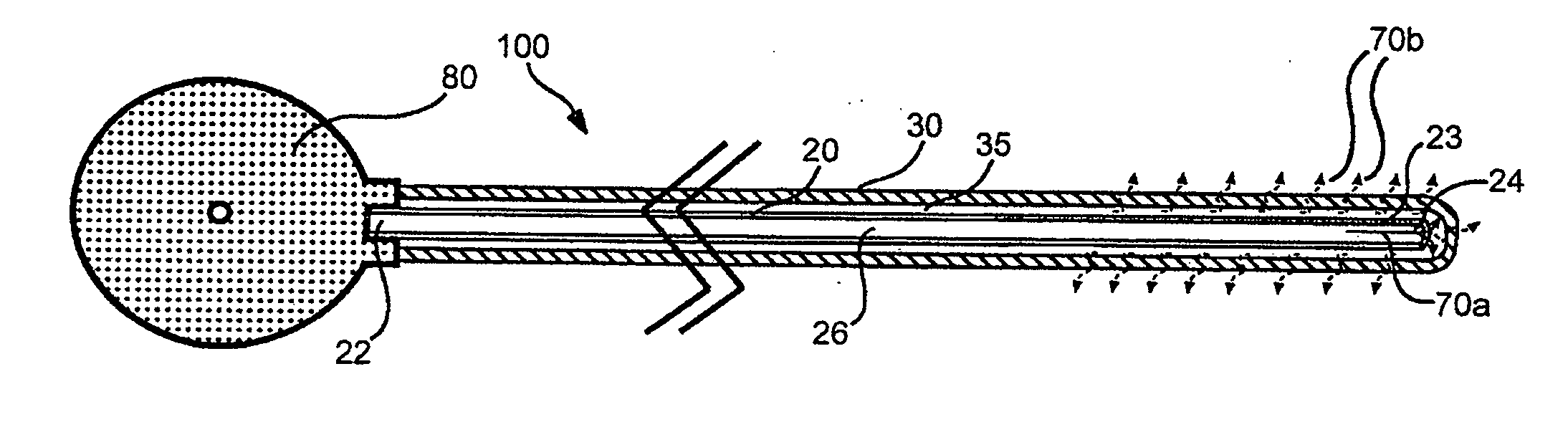
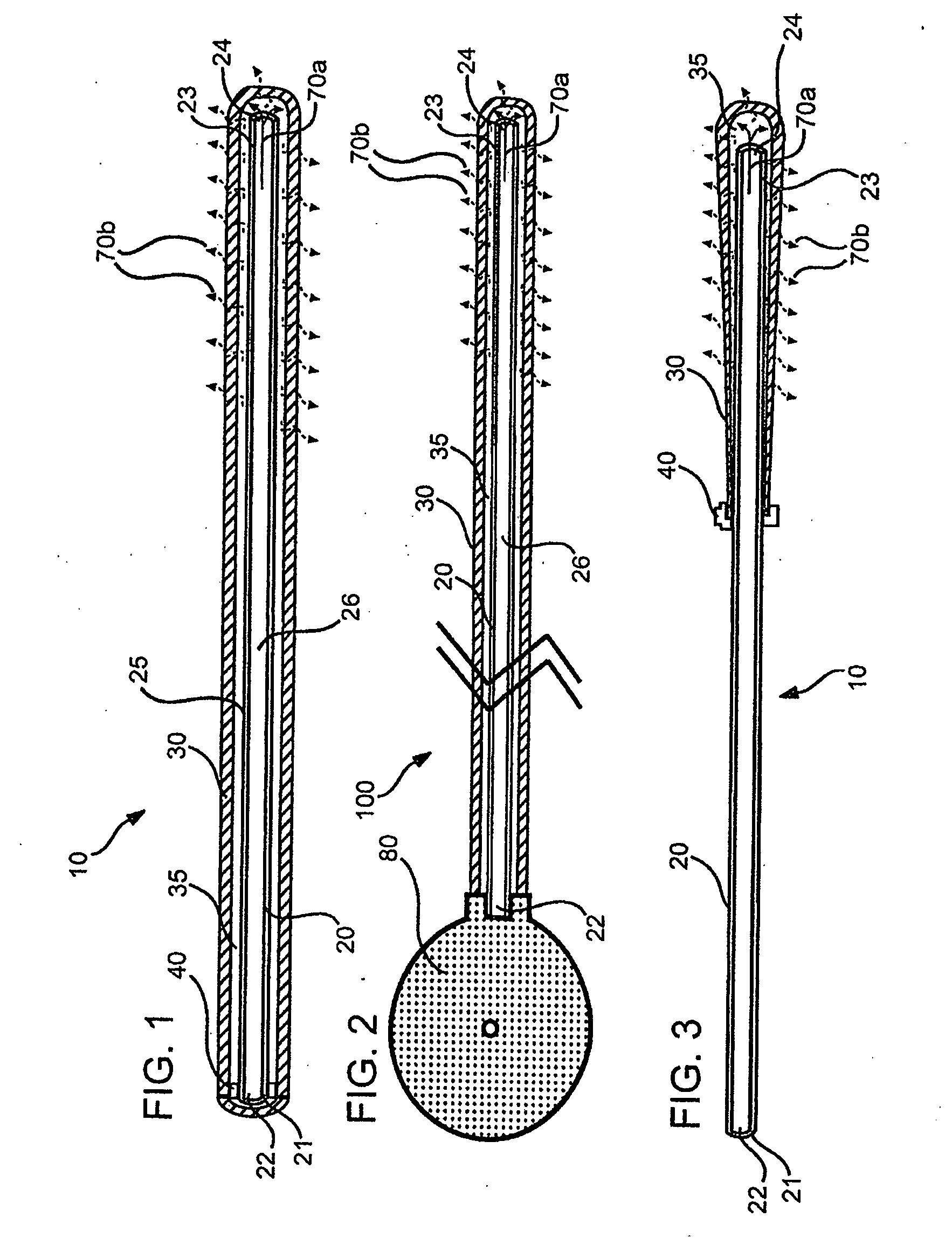
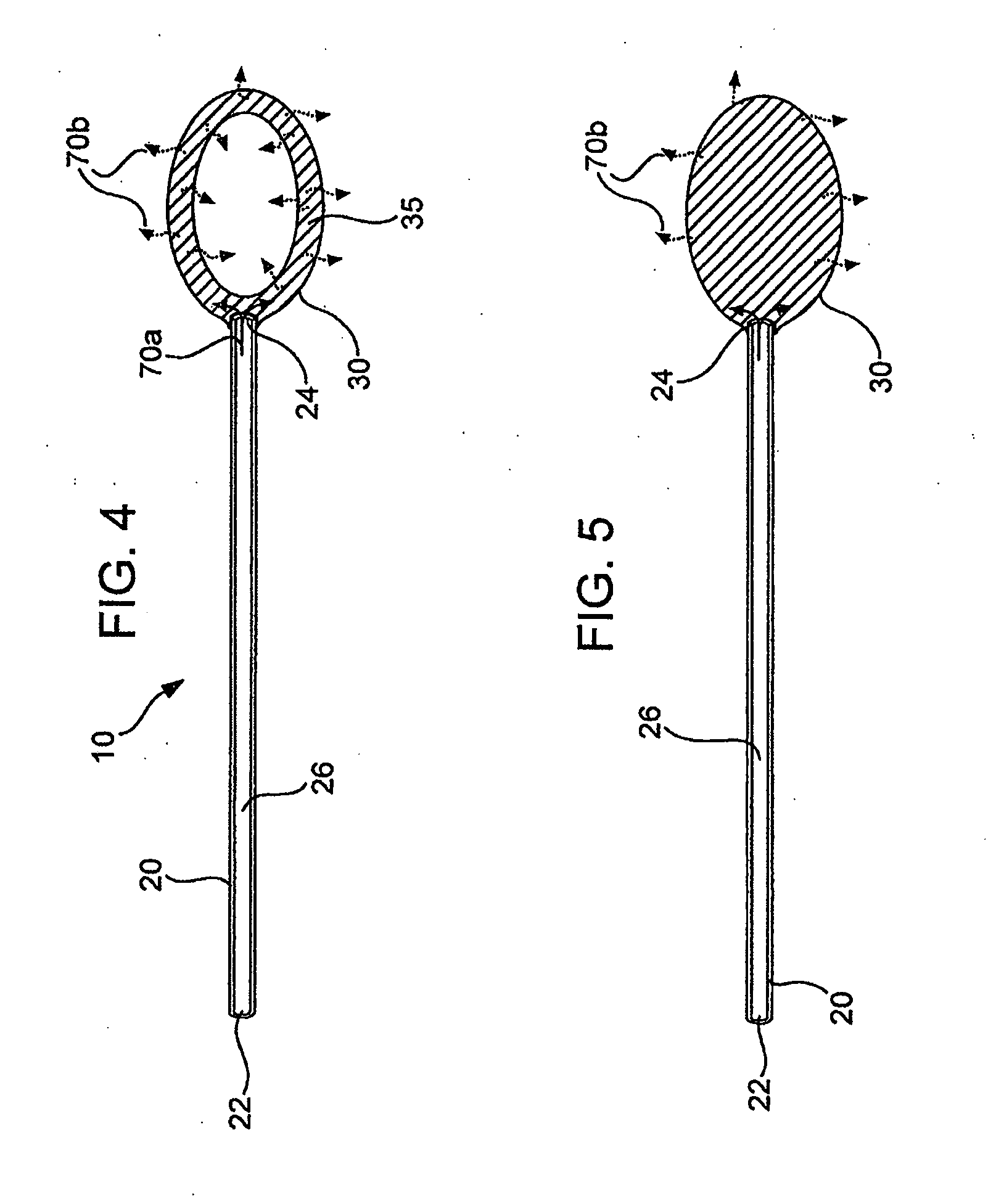
Local concentration management system
a concentration management system and concentration management technology, applied in the field of local concentration management system, can solve the problems of limited access to treatment sites, difficulty in site-specific drug delivery, and limited number of limitations, and achieve the effect of convenient handling, low volume, and extremely small volum
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Polymeric Cup as Diffuser Element
[0139] Polymeric film is shaped into a diffuser element having a “cup” or “hat” configuration, and is placed over an exit outlet of a drug delivery device, such that the elongate body of the device is actually contained within the body of the drug delivery device (FIG. 8). The diffuser element serves as a secondary rate control means, and capture bursts of formulation that may be released from the drug delivery device.
[0140] The following is an estimate of normalized permeability of suitable polymeric materials for such polymeric diffuser elements for use in delivery of a sufentanil formulation from a drug delivery device, e.g., an osmotic pump. The following assumptions were made in calculating the permeablities of the polymeric materials: [0141] Minimum release rate of agent from device: 2.5 μg / hr [0142] Maximum release rate of agent from device: 20 μg / hr [0143] Diffuser element configuration and dimensions: a cylinder having a diameter of 0.3 cm...
example 2
Delivery of Baclofen HCl
[0149] A Baclofen HCl solution in DMSO (335.8 mg / cc) was delivered using a prototype of a catheter of the invention. With reference to the equations described herein, the dilution ratio was calculated, with Co=335.8 mg / cc Baclofen, with KΠo=31.3:1 mils / cm2 hr for the Silastic tubing (Dow Corning Q 4750). The outer diameter of the tubing (OD) was 0.047 in (0.0597 cm), while the inner diameter was inner diameter (ID) was 0.025 in (0.0317 cm). The mean diameter (calculated by taking the log average value due to variations in wall thickness) was 0.0443 cm, with a tubing wall thickness of 11 mils (0.0235 in minus 0.0125 in). The pumping rate was 20 μl / day (0.083 μl / hr). The length of the elongate body catheter was 5.22 cm.
[0150] Using these values and Equation (2) above, Φo is calculated to be 4.13 :l / hr. Using this value in Equation (3), the estimated flow rate at the exit of the Silasitc tubing (elongate body) should be Fι=0.083 (1+2×4.13 / 0.083)1 / 2=0.83:l / hr. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com