Implantable myocardial ischemia detection, indication and action technology
a technology of myocardial ischemia and implantable myocardium, which is applied in the direction of catheters, instruments, therapy, etc., can solve the problems of local ischemia or infarction of the heart muscle, chronic with continuously evolving symptoms and severity, and impaired heart performance, so as to reduce the ability to perfuse the heart muscle and reduce the effect of mechanical performan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0040] Certain embodiments of the invention pertain to methods and devices for detecting ischemia or infarction, diagnosing, alerting the patient and / or treating the ischemic heart disease.
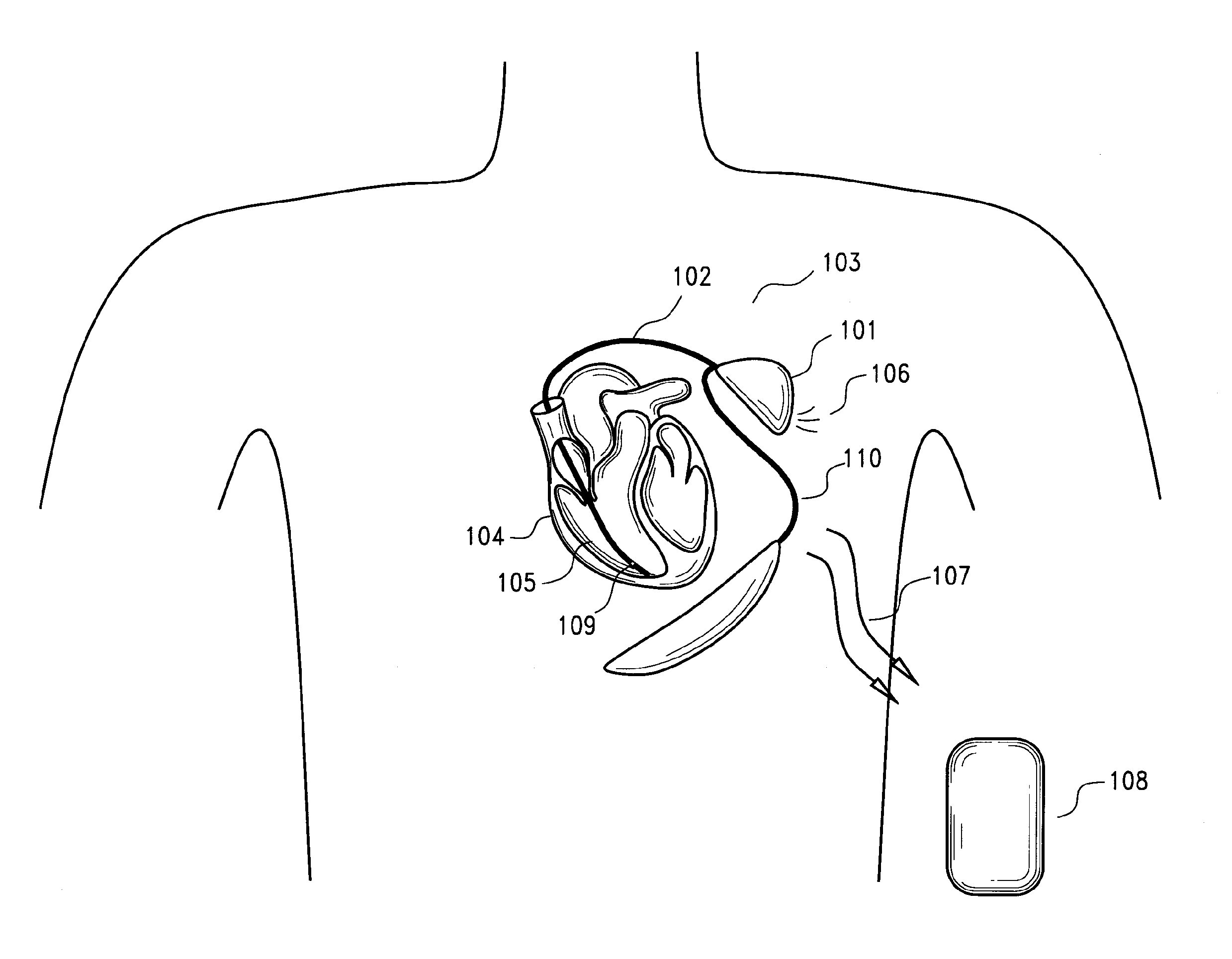
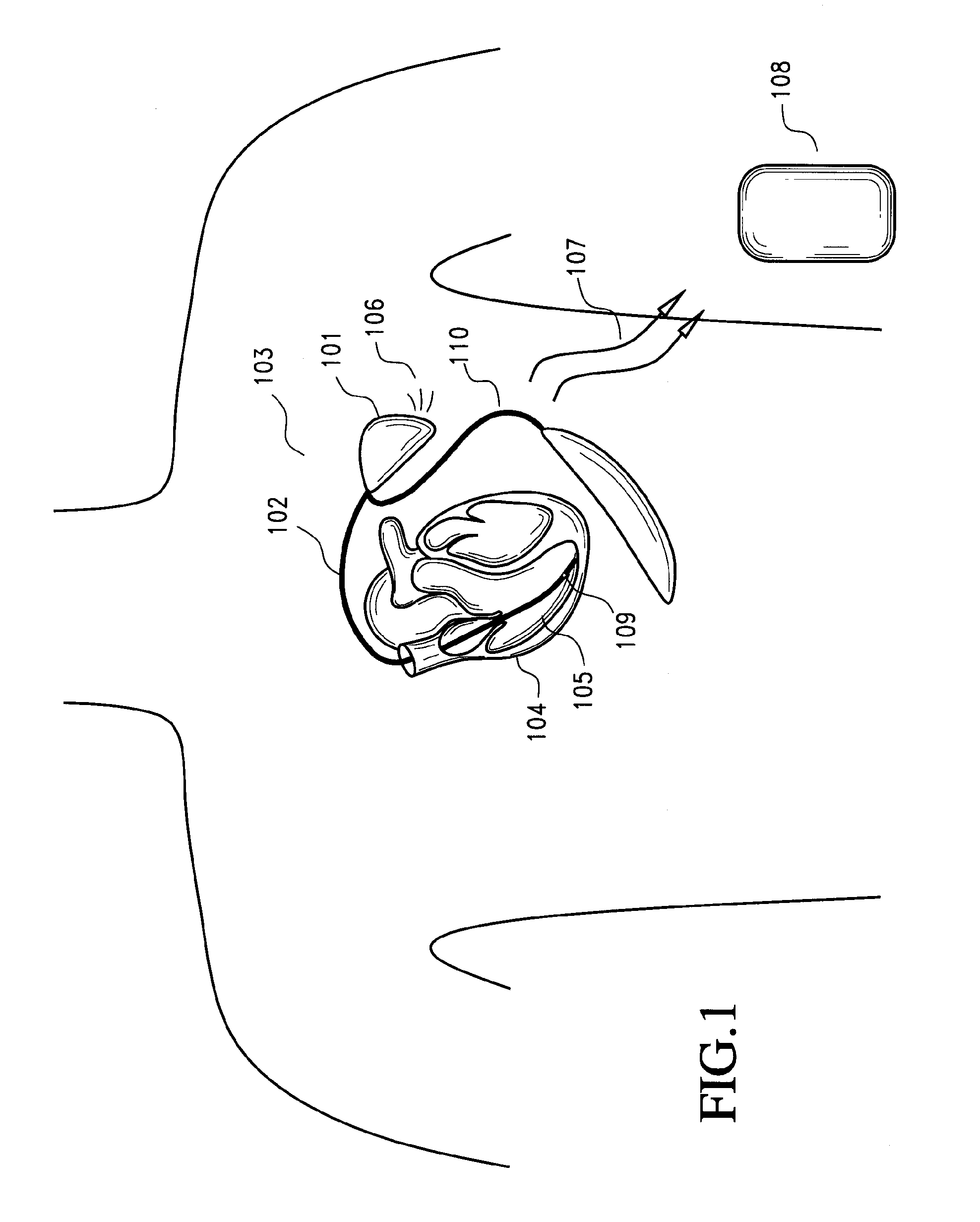
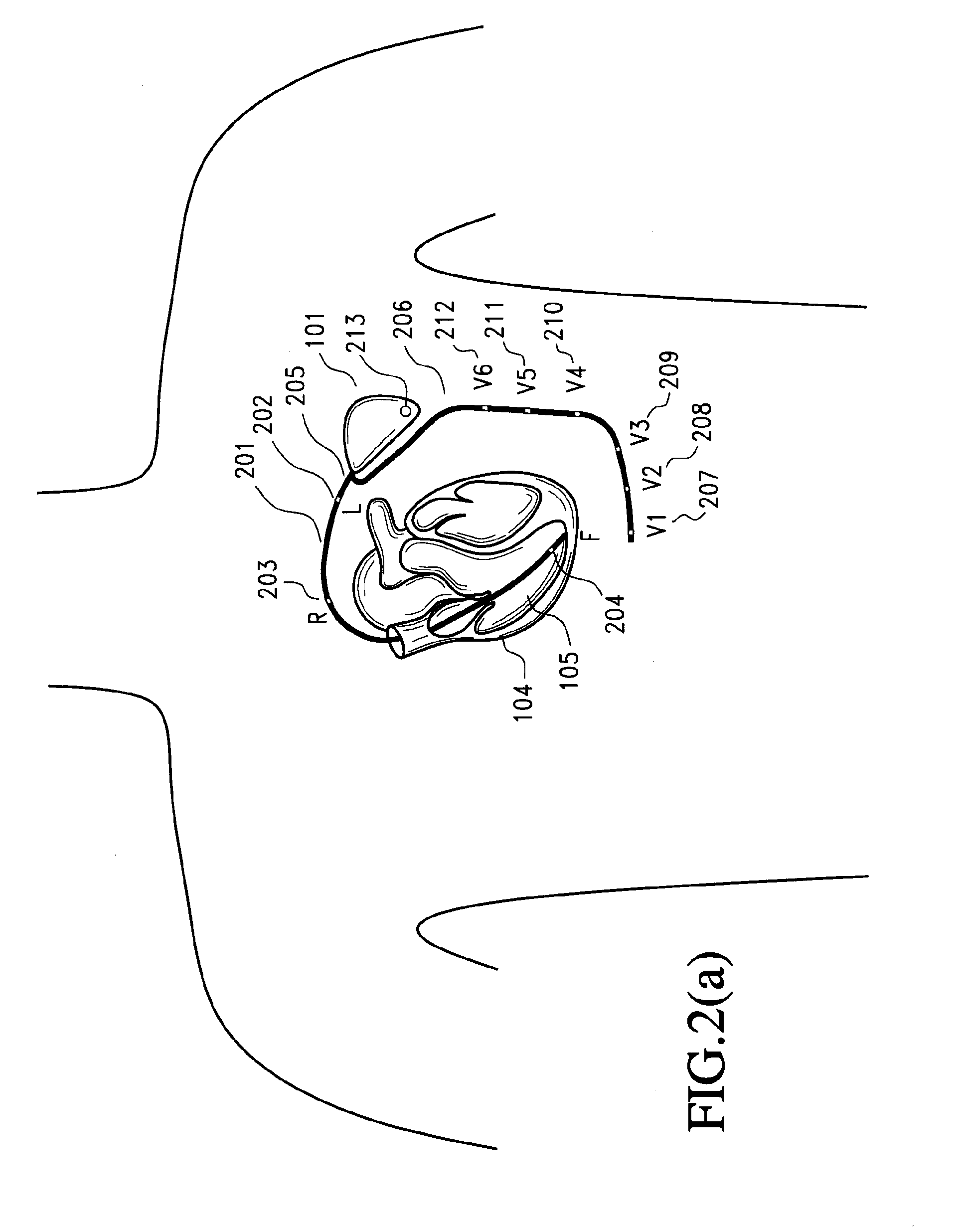
[0041] Implantable myocardial ischemia and / or infarction (MI / I) detection technology according to certain preferred embodiments is illustrated in FIG. 1. Embodiments include methods and devices to detect and treat MI / I. One embodiment of a method includes: i) placement / implantation of the device inside the chest or other body cavity, ii) placement and implantation of electrodes and sensors to selected areas of the myocardium, iii) connection of one or more of the electrodes and sensors to the implanted device, iv) detection of an ischemic event by the analysis of the EGM signal and sensor data, v) the method of analysis of the EGM signal using signal processing means in time and frequency domain, vi) communicating the stored EGM signals to an external device using telecommunication means, vii) al...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com