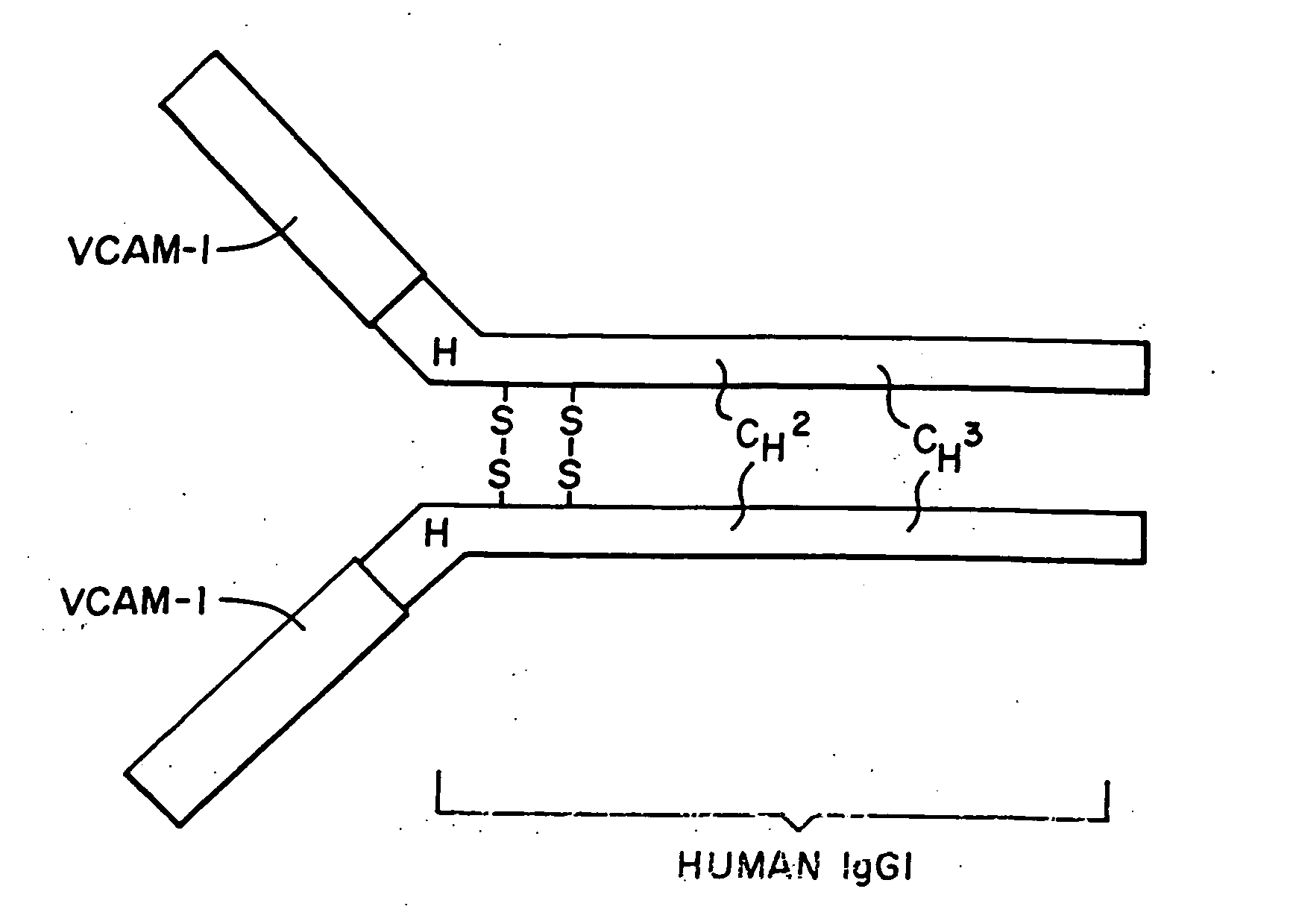
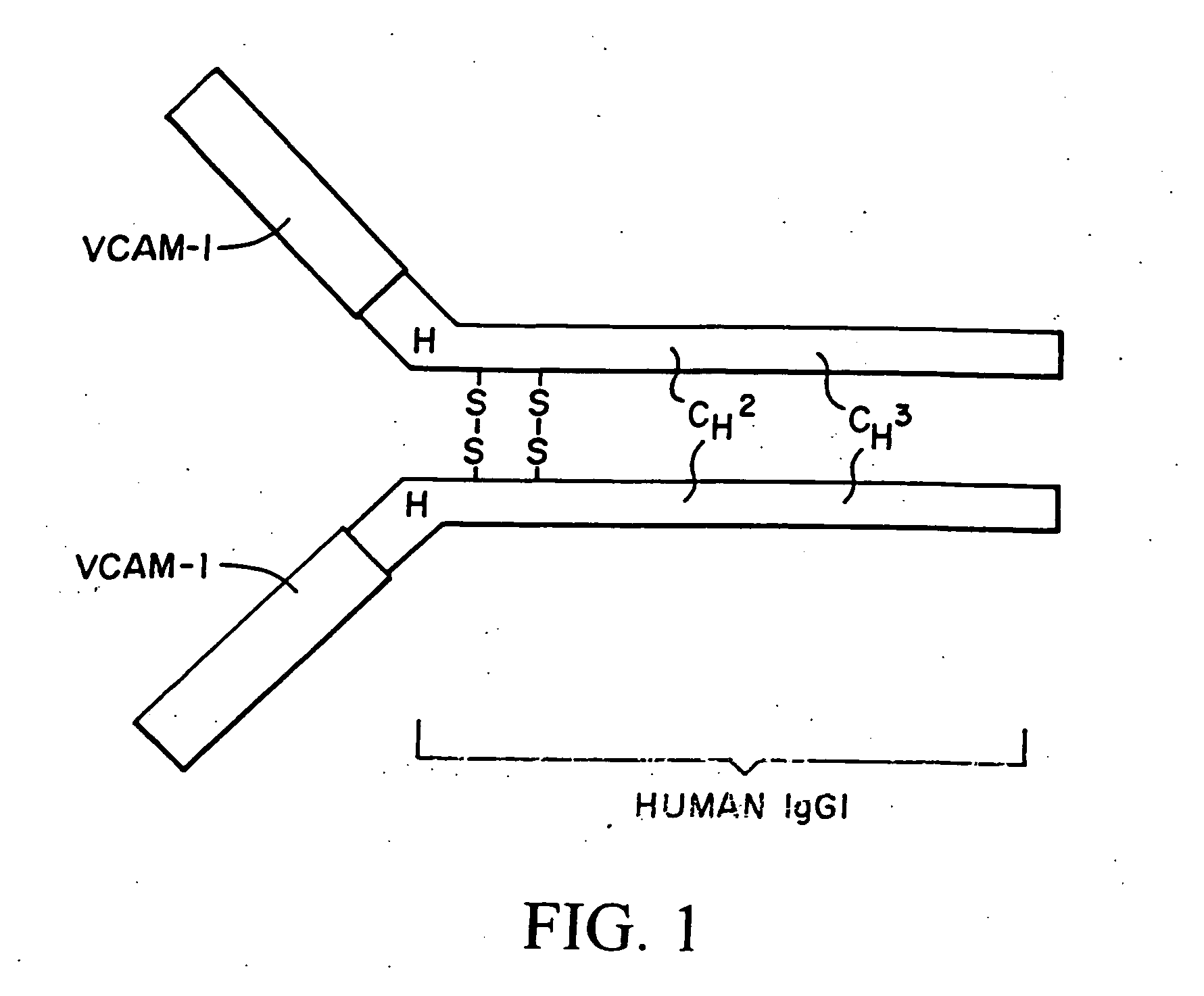
Treatment for inflammatory bowel disease
a technology for inflammatory bowel disease and treatment, which is applied in the direction of anti-immunoglobulin, therapy, peptide/protein ingredients, etc., can solve the problems of infection or bowel obstruction, ibd has no cure, and ibd is not yet understood
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example i
VCAM1 Expression in the Colon
[0048] Experiments were performed to determine whether active IBD involved the expression of endothelial cell surface proteins involved in leukocyte adhesion. Expression of VCAM-1 in colon tissue of IBD sufferers and normal or uninvolved colon tissue controls was evaluated. Human colonoscopic biopsy tissue samples were obtained, with informed consent, and prepared as frozen sections by mounting in OCT compound (TissueTek) and quick freezing in isopentane / liquid nitrogen. The human colon samples were from normal colon, active ulcerative colitis colon (UC-active), inactive ulcerative colitis colon (UC-inactive), uninvolved ulcerative colitis colon (UC-uninvolved), active Crohn's Disease colon (CD-active), and uninvolved Crohn's Disease colon (CD-uninvolved).
[0049] Frozen sections (˜4μ) were placed on gelatin-coated slides (1% gelatin, heated at 60° C. for 1-2 min., air dried, 1% formaldehyde at room temp., air dried), air dried 30 minutes, fixed in acet...
example ii
Anti-VLA-4 Antibody Recognition of CTT White Blood Cells
[0052] An anti-VLA-4 monoclonal antibody (HP1 / 2, obtained from Biogen, Inc., Cambridge Mass.) was tested to confirm that it recognized an epitope on CTT leukocytes.
[0053] Blood samples (3 ml) from CTTs were heparinized and the CTT peripheral blood mononuclear leukocytes (PBLs) were isolated using a Ficoll-Hypaque gradient (Pharmacia) according to the manufacturer's instructions for isolation of human PBLs. CTT PBLs were examined for their ability to bind to the murine anti-human VLA-4 monoclonal antibodies HP1 / 2 and HP2 / 1 by FACS analysis using a Becton Dickenson FACStar and standard techniques (see, e.g., Lobb et al., 1991a [31]). Both monoclonal antibodies bound to CTT PBLs, indicating that both human and CTT VLA4 have similar epitopes recognized by these two antibodies.
[0054] CTT PBLs were also observed to adhere to microtiter plates coated with immobilized recombinant soluble human VCAM-1 (Biogen, Inc.), which binding w...
example iii
Cotton Top Tamarin Trials
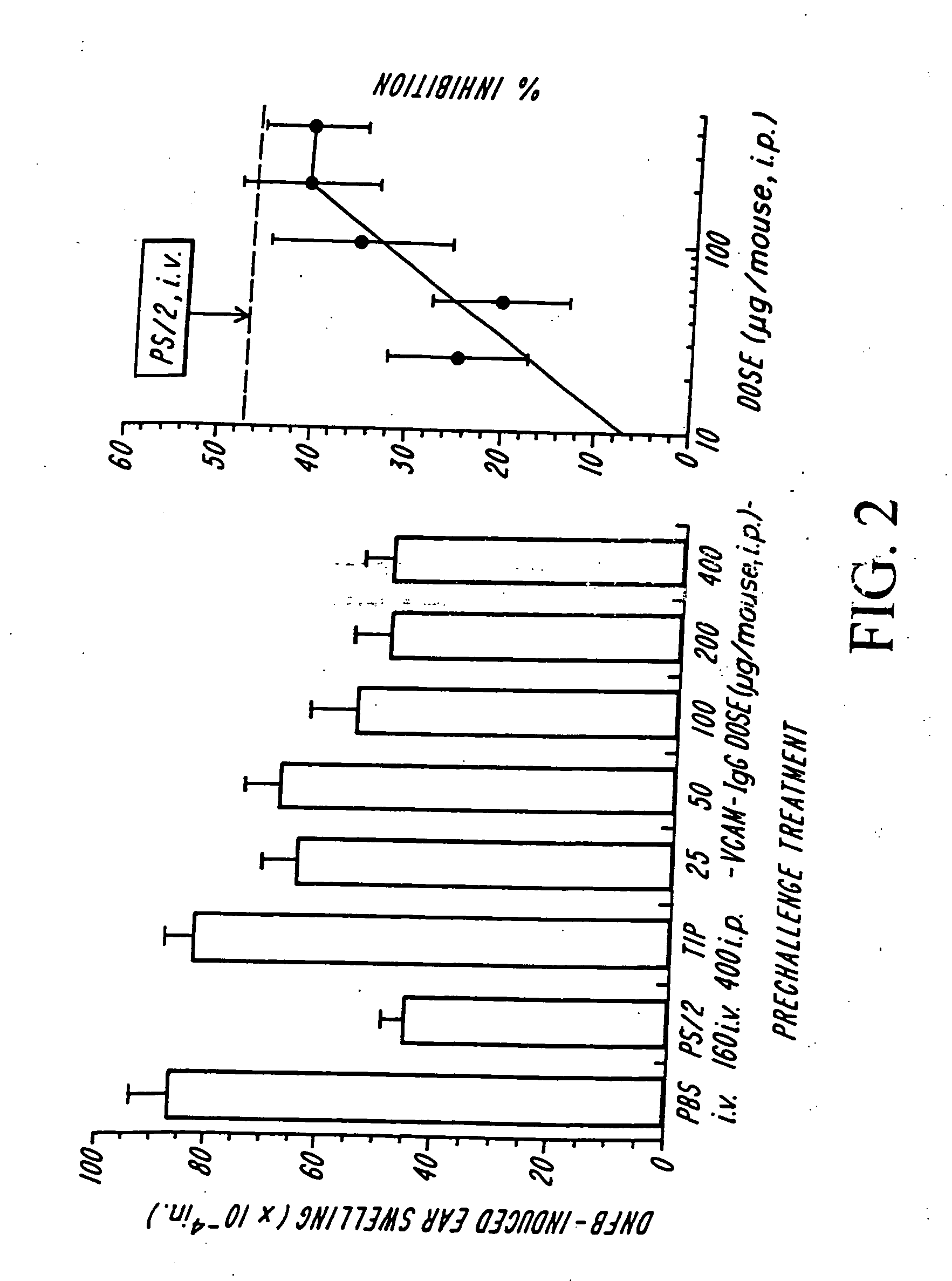
[0055] A stock solution in sterile saline of the Anti-VLA-4 antibody, HP1 / 2 (IgG1), and a placebo control (saline only), were prepared for administration to ten cotton top tamarins (CTTs) exhibiting symptoms of spontaneous colitis (i.e., diarrhea, etc.; see, Madara et al. [18]). Five CTTs received HP1 / 2 and five received placebo, by intravenous injection. The CTTs receiving HP1 / 2 were injected with 1 mg HP1 / 2 per day (i.e., about 2 mg / kg / day, based on approximate half-kilogram weight of a CTT) for eight days (on Days 0, 1, 2, 3, 4, 5, 6, and 7 of the trial). Colon tissue samples obtained from the animals were biopsied every other day (on Days 0, 2, 4, 6, 8, and 10 of the trial).
[0056] Data from the biopsies were used to determine an acute inflammation index for each animal, giving a semi-quantitative analysis of the course of the colitis. (See, Madara et al. [18].) The inflammation indices before the trial began (Day 0) and at the end of the trial at Day ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| soluble | aaaaa | aaaaa |
| solubility | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com