Exclusion Device and System For Delivery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
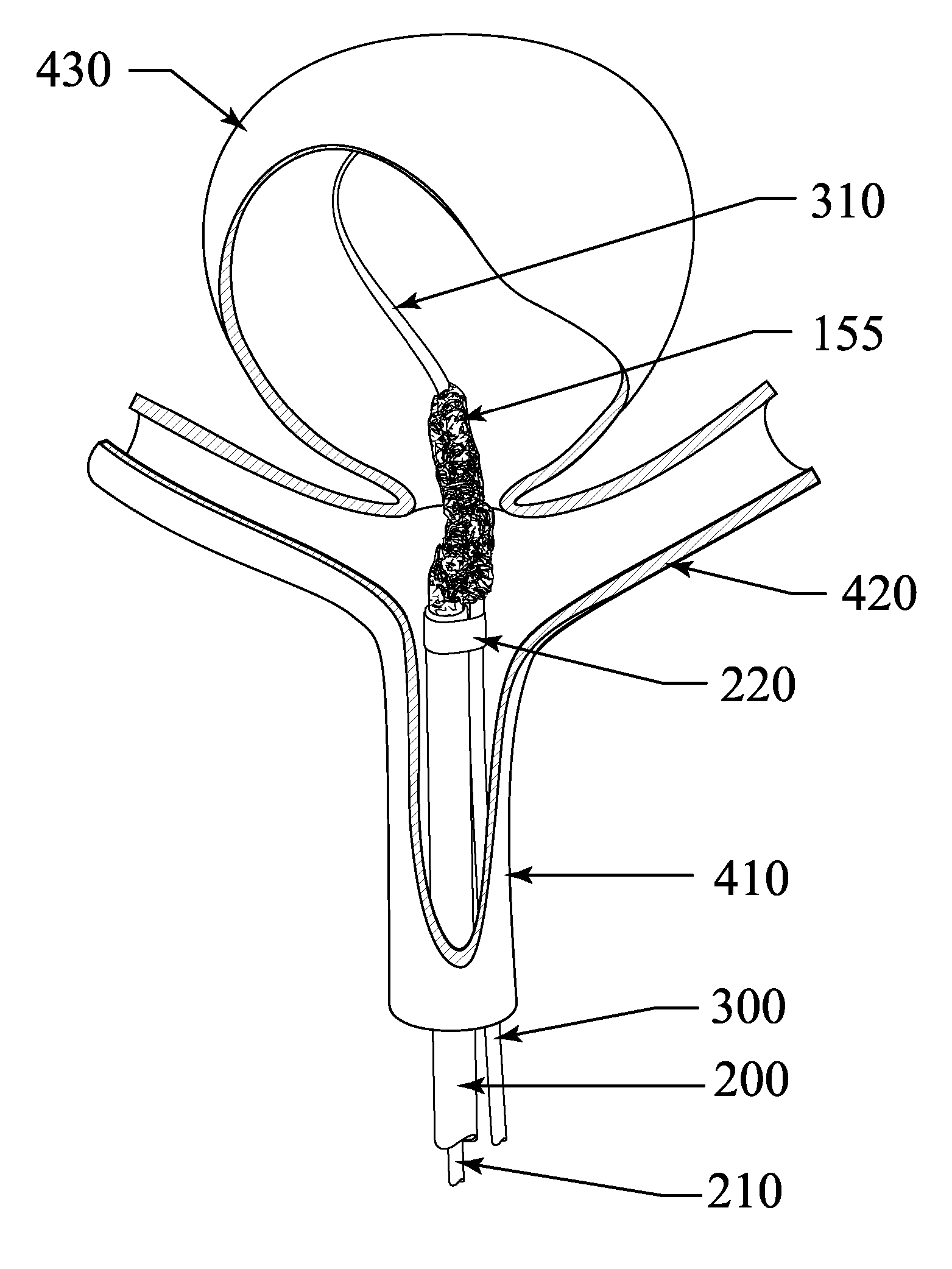
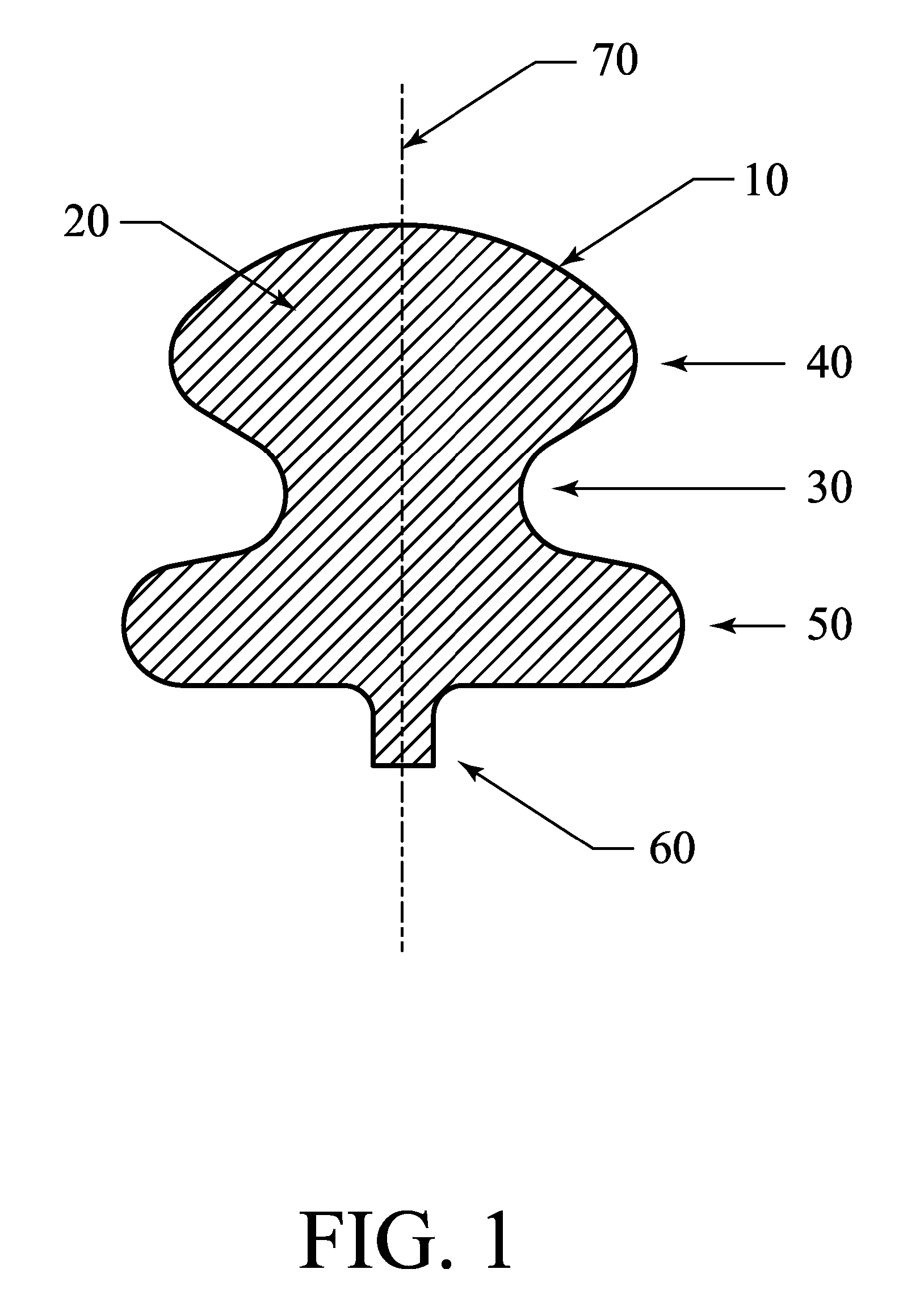
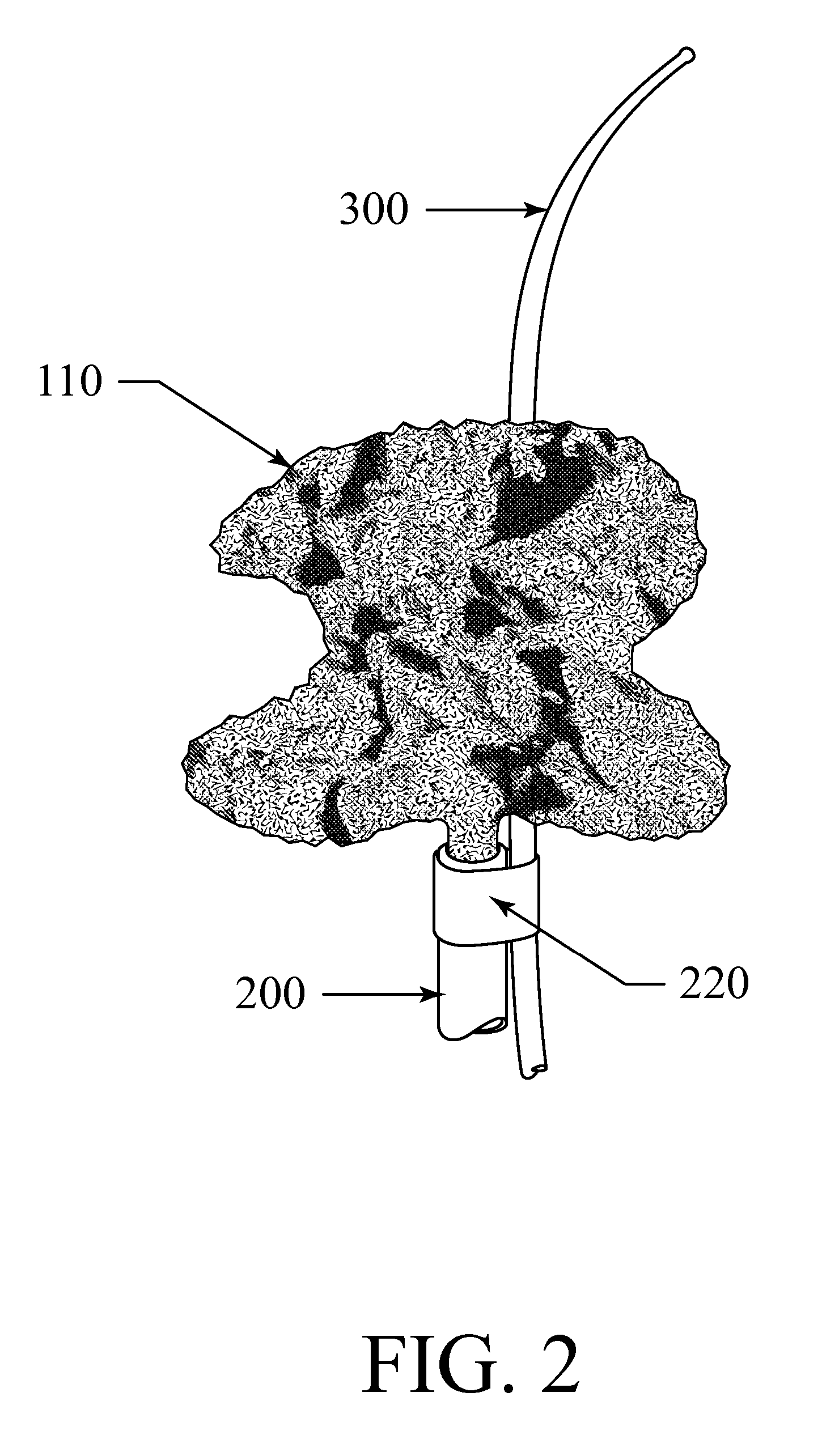
[0081] The current invention provides an exclusion device and novel catheter-based endovascular delivery and deployment methods. In the illustrative embodiments depicted in FIGS. 1-18, an exclusion device 10 is delivered to an aneurysm 430, positioned at the neck 435 of the aneurysm, and deployed, thereby blocking the neck of the aneurysm and reducing blood flow into the aneurysm 430. The deployment leaves the parent (proximal) artery 410 fully open.
[0082] At a bifurcation, the proximal artery 410 splits into two smaller arteries 420 as shown in FIGS. 4-8, and FIGS. 11-18. The deployed exclusion device does not block side branch arteries that may exist near the aneurysm. Aneurysms at bifurcations (as shown in FIGS. 4-8 and FIGS. 11-18) and aneurysms on the side of an artery (as shown in FIG. 10) may be treated. The exclusion device, deployed to cover the neck of an aneurysm, reduces blood flow into the aneurysm and triggers a thrombus in the aneurysm that starts the healing process...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com