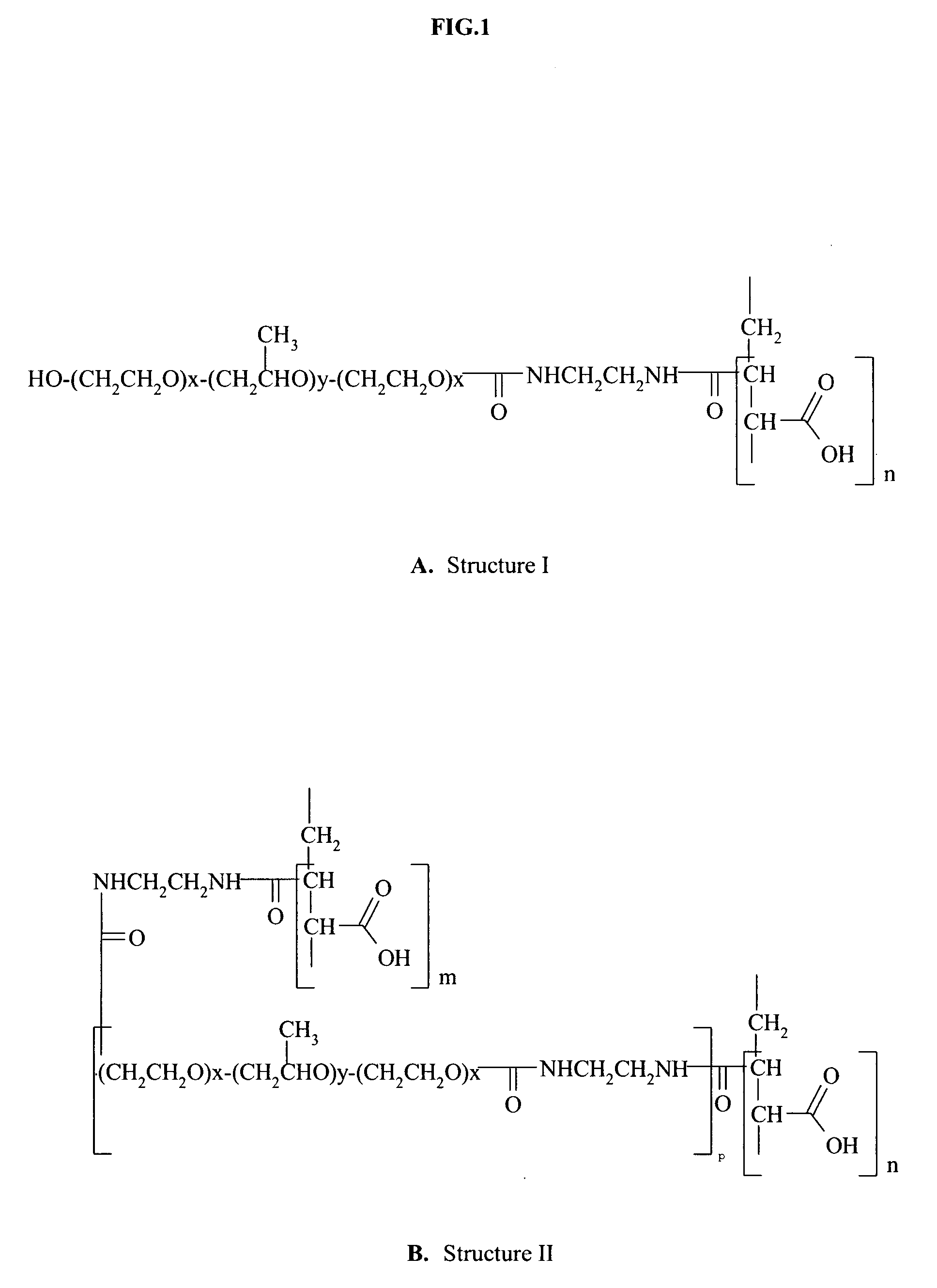
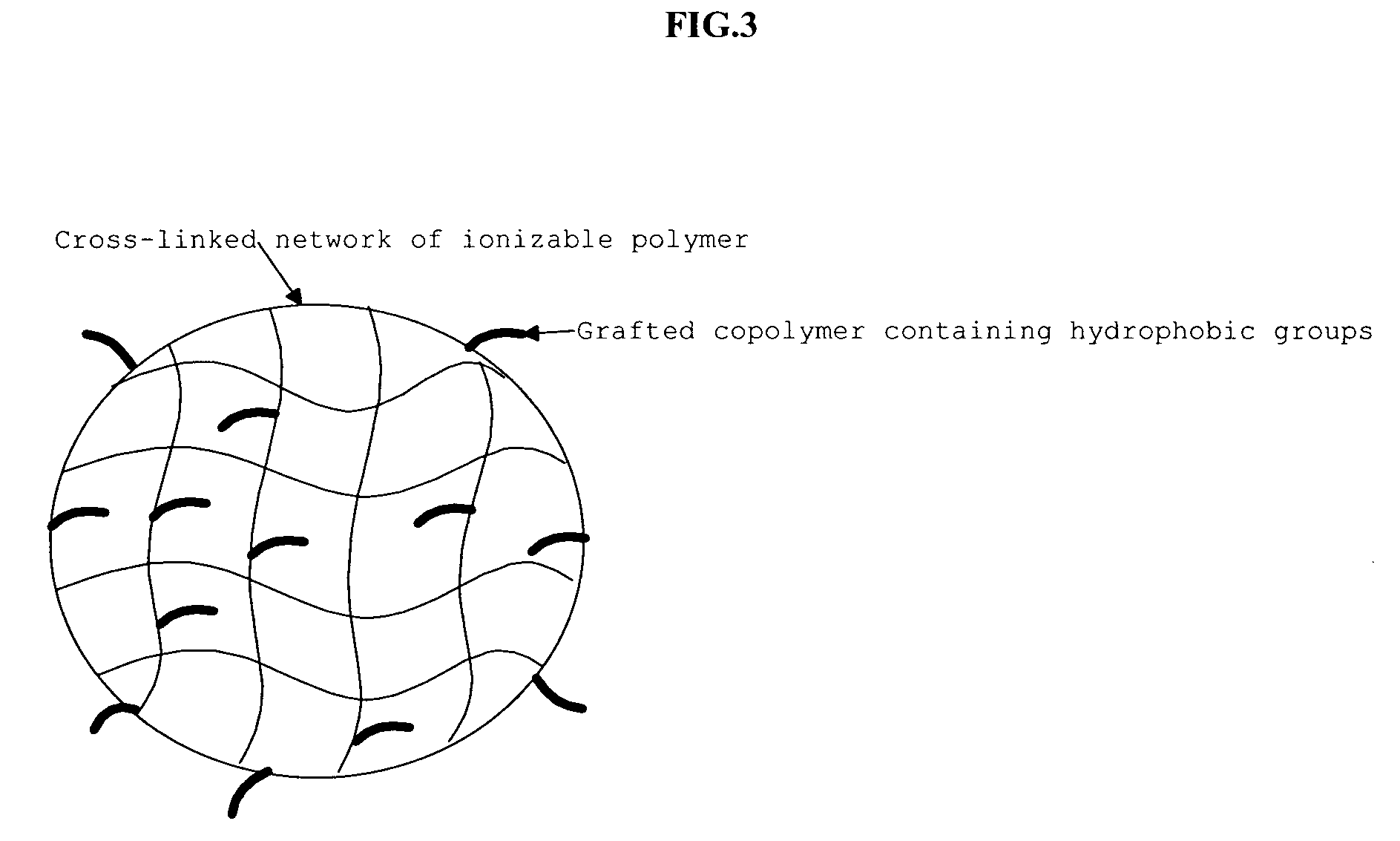
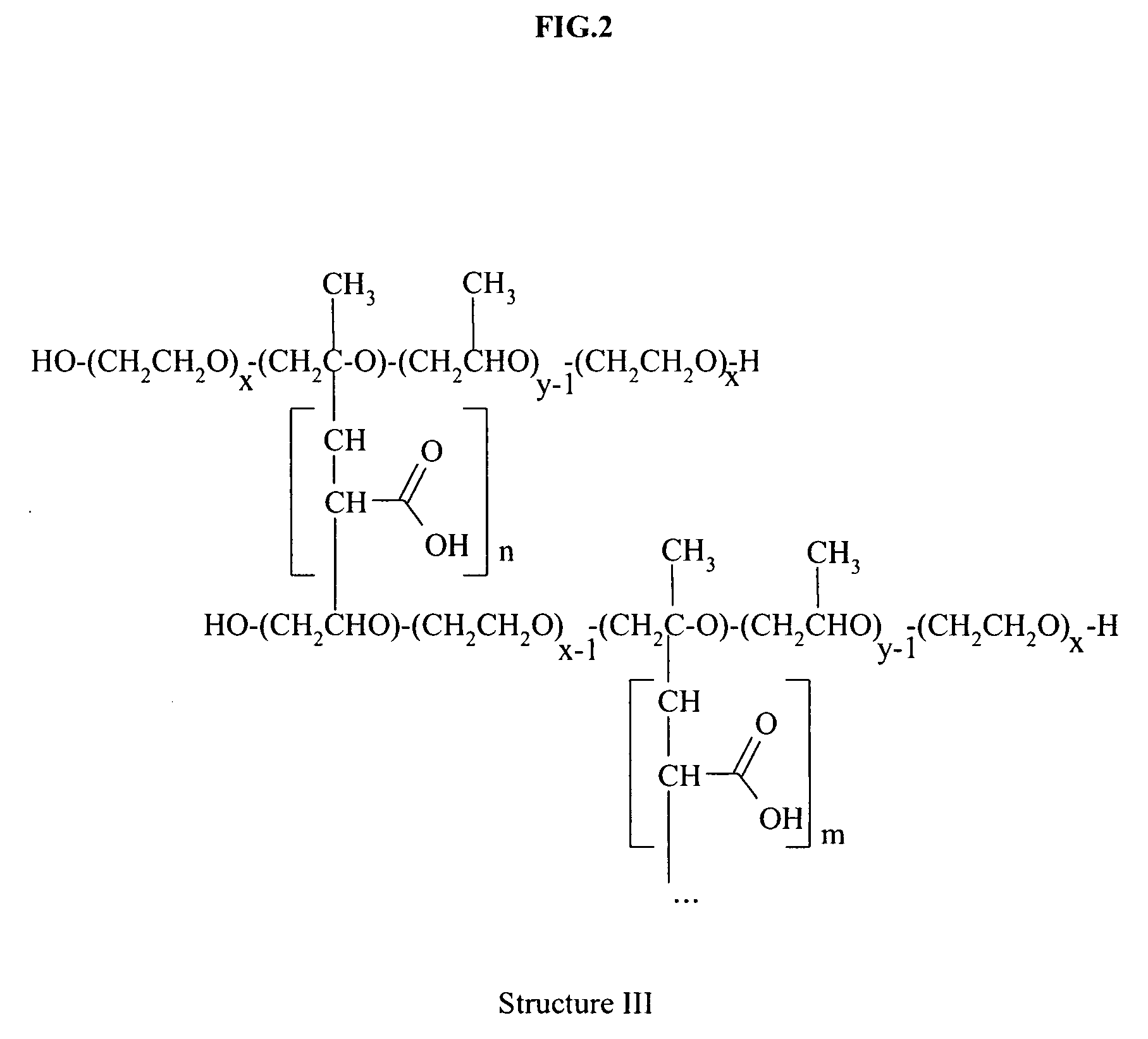Responsive microgel and methods related thereto
a microgel and responsive technology, applied in the field of microgels, can solve the problems of toxic poly(alkylacrylamide), creating ion-sensitive microgels, chemical moieties (amides or other) required to achieve the linkage between an amphiphilic copolymer such as polyether and the polyelectroly
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example i
Drug Release from Responsive Microgels of Polyether-Modified Poly(Acrylic Acid)
[0146] PLURONIC® F127 NF, L92 and L61 were obtained from BASF Corp. (Mount Olive, N.J.) and used as received. The properties of these PLURONIC® surfactants are presented in Table 1:
TABLE 1Properties of the PLURONIC ® surfactants used in thisstudy. M. Yu. Kozlov, Macromolecules, 2000, 33, 3305-3313; M. J.Kositza, et al., Langmuir, 1999, 15, 322-325; BASF Catalog.NumberNumberHydrophilic-Cloud pointCritical micelleNominalof POof EOlipophilicin water at 1concentration atCopolymerMWunitsunitsbalancewt %, ° C.25° C., ML6120003063321.1 × 10−4L92365060166698.8 × 10−5F127126006520022>1002.8 × 10−6
[0147] Materials
[0148] A fluorescent dye, 5-(4,6-dichlorotriazinyl)aminofluorescein (DCTAF, 99%) was obtained from Molecular Probes, Inc. (Eugene, Oreg.). Acrylic acid (99%, vinyl monomer), ethylene glycol dimethacrylate (98%, divinyl cross-linker, EGDMA), dodecane (99+%, solvent), 4,4′-azobis(4-cyanovaleric acid) (75...
example ii
Microgel Synthesis
[0168] Nonionic copolymer PLURONIC® F127 NF was obtained from BASF Corp. and used without further treatment. It has a formula EO100PO65EO100, nominal molecular weight 12600, molecular weight of PPO segment 3780, 70 wt % of EO, and cloud point above 100oC. Acrylic acid (99%) (vinyl monomer), ethylene glycol dimethacrylate (EGDMA) (98%) (divinyl cross-linker), dodecane (99+%) (solvent), and 4,4′-azobis(4-cyanovaleric acid) (75+%) (azo initiator) were purchased from Aldrich Chemical Co. and used as received. Lauroyl peroxide (97%) (redox initiator) was obtained from Fluka Chemie AG, Switzerland. Poly(vinylpyrrolidinone-co-1-hexadecene) (Ganex V-216) (dispersion stabilizer) was obtained from International Specialty Products and used as received. All other chemicals, gases and organic solvents of the highest purity available were obtained from commercial sources.
[0169] Synthesis was carried out on a laboratory scale in an adiabatic mode. Acrylic acid (vinyl monomer) (...
example iii
Microgel Superabsorbent Properties
[0171] The ability of microgels to absorb water was estimated using a volumetric method. Single microgel particles were placed into glass capillary tubes (internal diameter 1-1.2 mm) using suction pressures applied by an Ultramicro Accropet filler / dispenser via rubber connector. The tubes were placed into a homemade glass thermostatted cuvet and observed under an inverted microscope equipped with a microscaler and a video monitor. Similar experimental setup was described in Eichenbaum, G. M., et al., Macromolecules, 1998, 31, 5084-5093. The boundaries of the spherical particles were fitted with the microscaler and a particle diameter was measured with an accuracy of ±0.5 μm or better. Initially, a diameter of a dry particle (do) was measured, then the capillary tube was gently filled with deionized water (pH adjusted by 5 M NaOH) immersed into a reservoir of the same solution. The diameter of the swollen particle (ds) was measured at a given temper...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com