Vaccination with immuno-isolated cells producing an immunomodulator
a technology of immunomodulator and immunoisolation cell, which is applied in the direction of snake antigen ingredients, viral antigen ingredients, cancer antigen ingredients, etc., can solve the problems of inability to use a simple approach in all vaccination settings, limited strategy, and inability to completely eliminate the drawbacks of the approach
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Testing the Secretion of Human GM-CSF Release by Various Cells Lines after Transfection
[0188] This example shows the ability of different cell lines to secrete human GM-CSF with and without being irradiated.
Transfection Methods:
[0189] Human GM-CSF cDNA was cloned into a retroviral vector (MFG) as described in the literature (Danos Olivier and Mulligan Richard 1988 PNAS Vol. 85 p6460-64; Jaffe Elizabeth et al. 1993 Cancer research Vol. 53 p2221-26).
[0190] The human GM-CSF is inserted in-frame into the retroviral vector. The MFG-hGM-CSF construct was sequenced in order to ensure correct in-frame cloning.
[0191] Using transient transfection technique with lipofectamine MFG-hGM-CSF construct was transfected into 293-gpg cells as described in the literature (Ory Daniel et al. 1996 PNAS Vol. 93 p 11400-06). With adequate selection, these cells produce pseudotyped retroviral particle containing the hGM-CSF gene. These viral particles are replication defective but can infect a wide ran...
example 2
Experimental Protocol Assessing the Efficacy of Onco-Maxi-Vax. Autologous Irradiated Tumour Cells+Encapsulated GM-Producers Cells. Pre-Clinical Development in Mouse Model
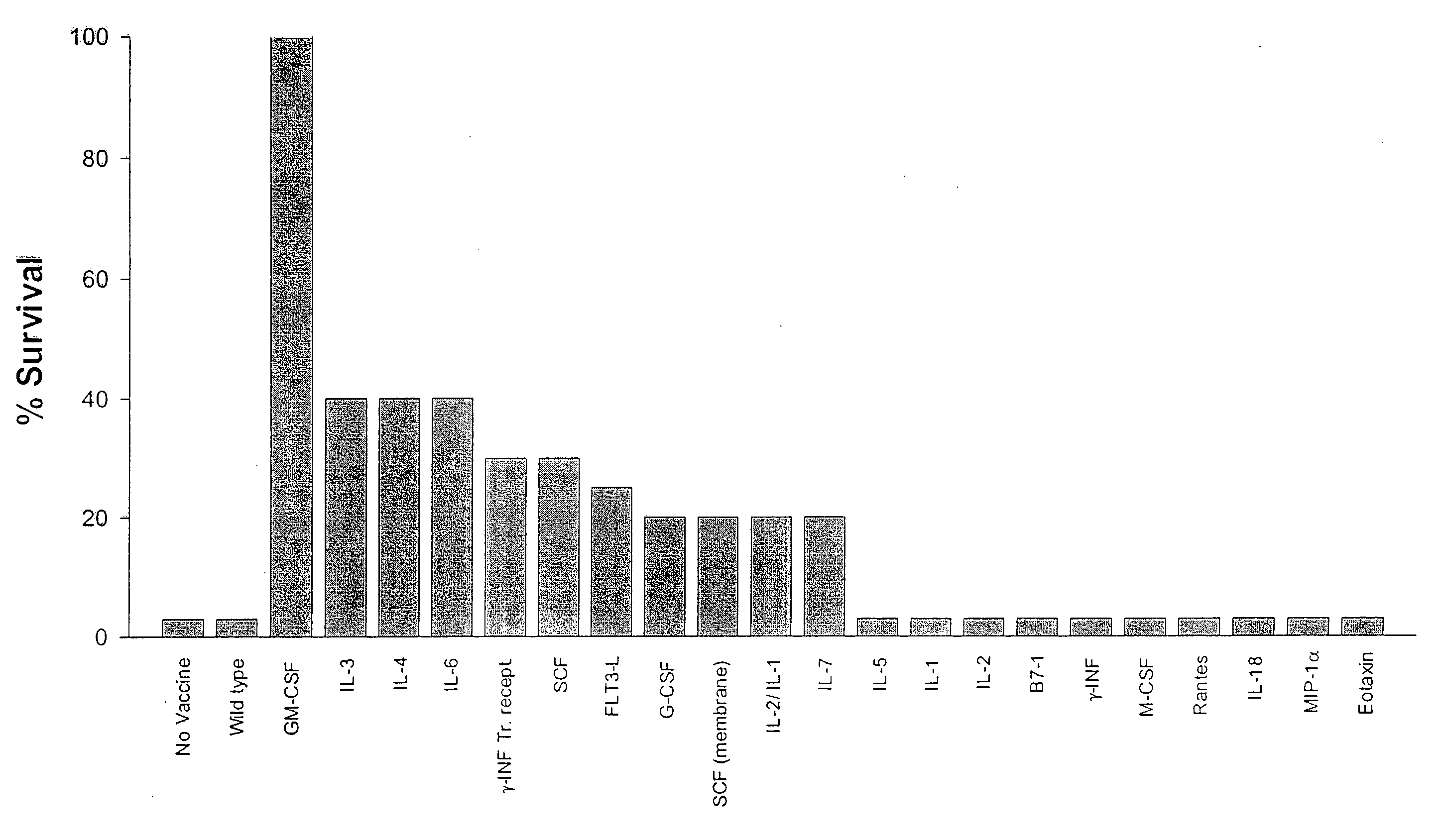
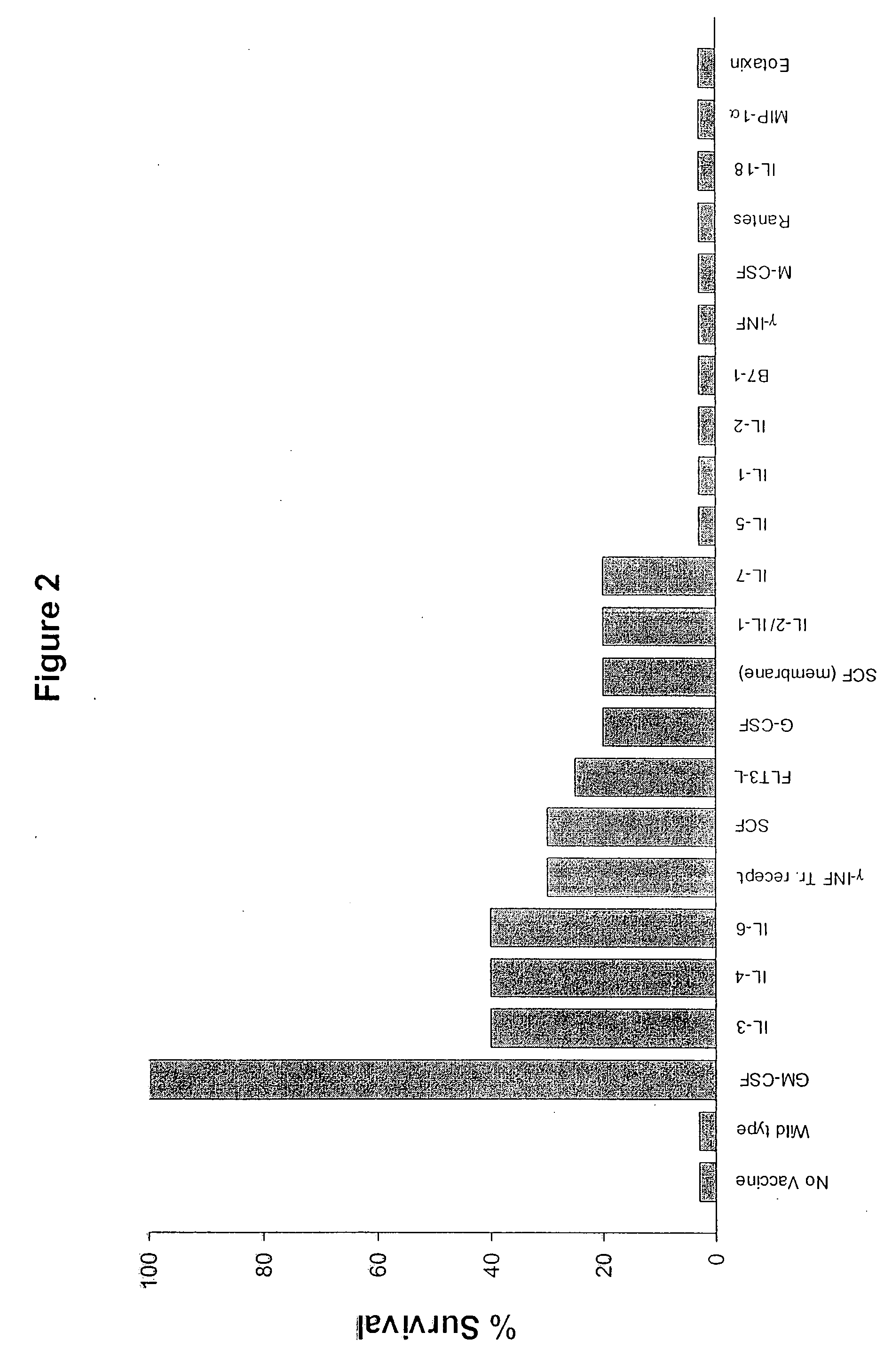
[0205] This example concerns the reproduction, using Onco-Maxi Vax, of the vaccination efficacy observed in the classical setting, when GM-CSF is produced by the irradiated tumour cells, in both wild-type mice and GM-CSF deficient mice. It also enables documentation of any new toxicity related to the use of the capsule, its manipulation or the cells it contains.
[0206] Furthermore, characterization of the response by standard techniques (Histology of vaccine site, cytokines profile, dendritic cell staining at the vaccination site) is carried out.
Experimental Design
A) Measurement of In-Vitro Release of Murine GM-CSF from the Capsule Containing GM-CSF Secreting Cells.
[0207] This is performed with standard monoclonal antibodies against murine GM-CSF in Enzyme-Linked immunoabsorbent Assays (ELISA). (R&D systems). ...
example 3
Protocol for the Preparation of “Onco-Maxi-Vax” for Use in Humans
[0226] This example concerns the preparation of Onco-Maxi Vax, for the vaccination of a human patient. The protocol gives detailed information regarding the preparation of the antigenic load, the generation of the immuno-isolated cytokine provider and the immunization with the two components from the Onco-Maxi-Vax.
[0227] Every step of the vaccine preparation for the two components (irradiated autologous cells and encapsulated GM-CSF producing cells) and the immunizations are performed according to clinical GMP guidelines.
1) Harvest of Autologous Tumor Cells (Antigenic Load)
[0228] A tumour mass (primary lesion or metastasis) from the patient to be treated is surgically harvested. An standard pathological examination is performed on a portion of the mass in order to confirm the malignant nature of the harvested material. It is then processed in order to obtain a single cell suspension. This is performed by both mech...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com