Adipose Tissue Stem Cells, Perivascular Cells and Pericytes
a technology of perivascular cells and stem cells, applied in the field of adipose tissue stem cells, perivascular cells and pericytes derived from adipose tissue, can solve the problems of affecting the use of such cells, affecting the quality of bone marrow, and affecting the ability of bone marrow to be harvested, so as to increase vascularity and enhance angiogenesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
embodiments
[0130]The present invention provides a perivascular cell, or pericyte, derived from adipose tissue. In one aspect, the cell is a precursor to a perivascular cell. The phenotype can be determined, inter alia, by positional fate, morphological characteristics, and other phenotypic characteristics such as cell-surface markers and biological functional characteristics such as increased microvascular density.
[0131]In one embodiment, the invention provides methods of identifying such cells and their precursors. In another embodiment, the invention provides methods of enriching populations of such cells. In one aspect, the methods of enriching such cells includes methods of inducing differentiation of the precursor cells.
[0132]In one embodiment, the present invention provides methods of increasing vascularity / vascular density in vivo using ASC derived perivascular precursor cell subpopulations. Such subpopulations may be derived from freshly derived adipose tissue or from cultured adipose ...
examples
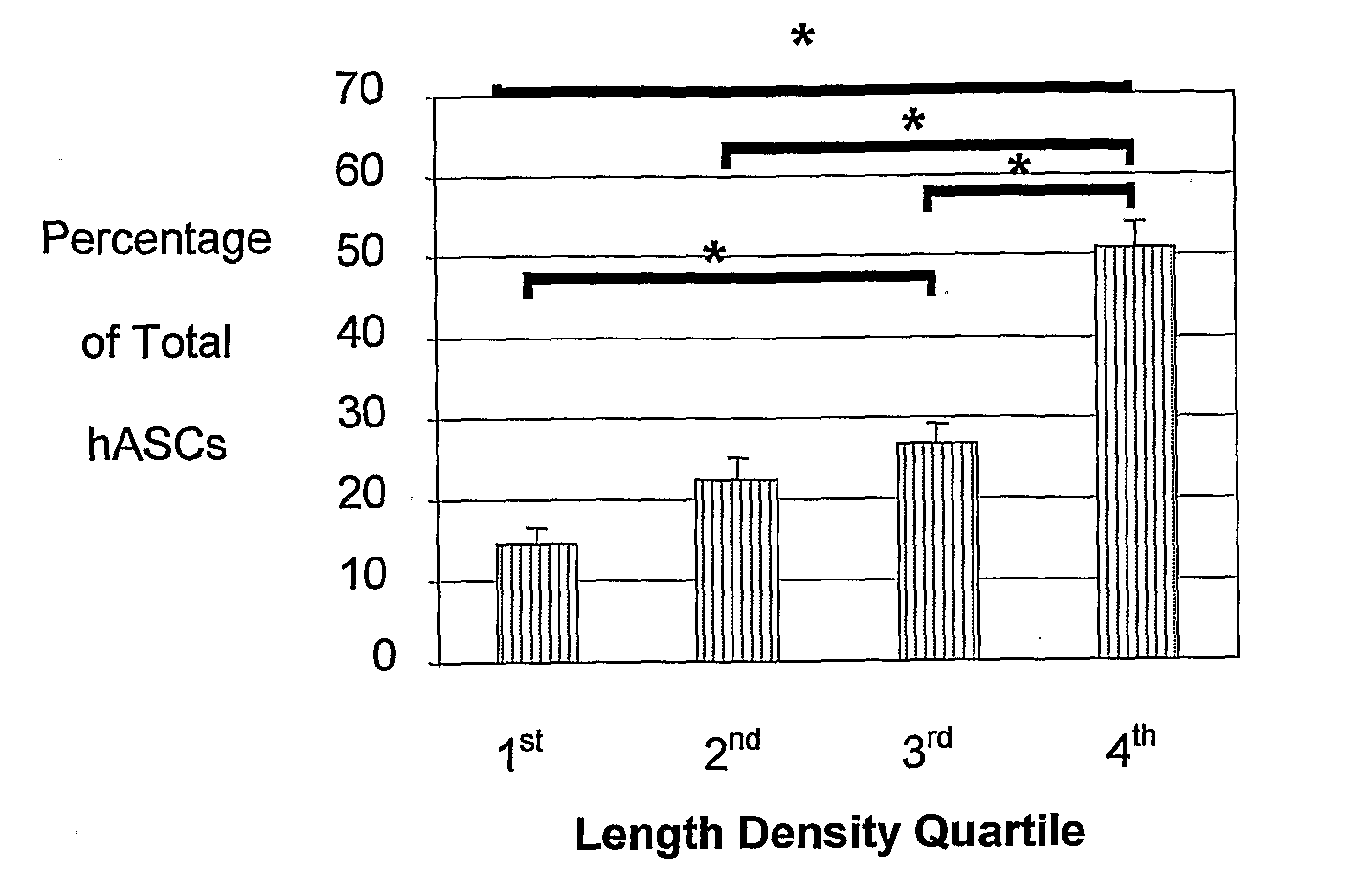
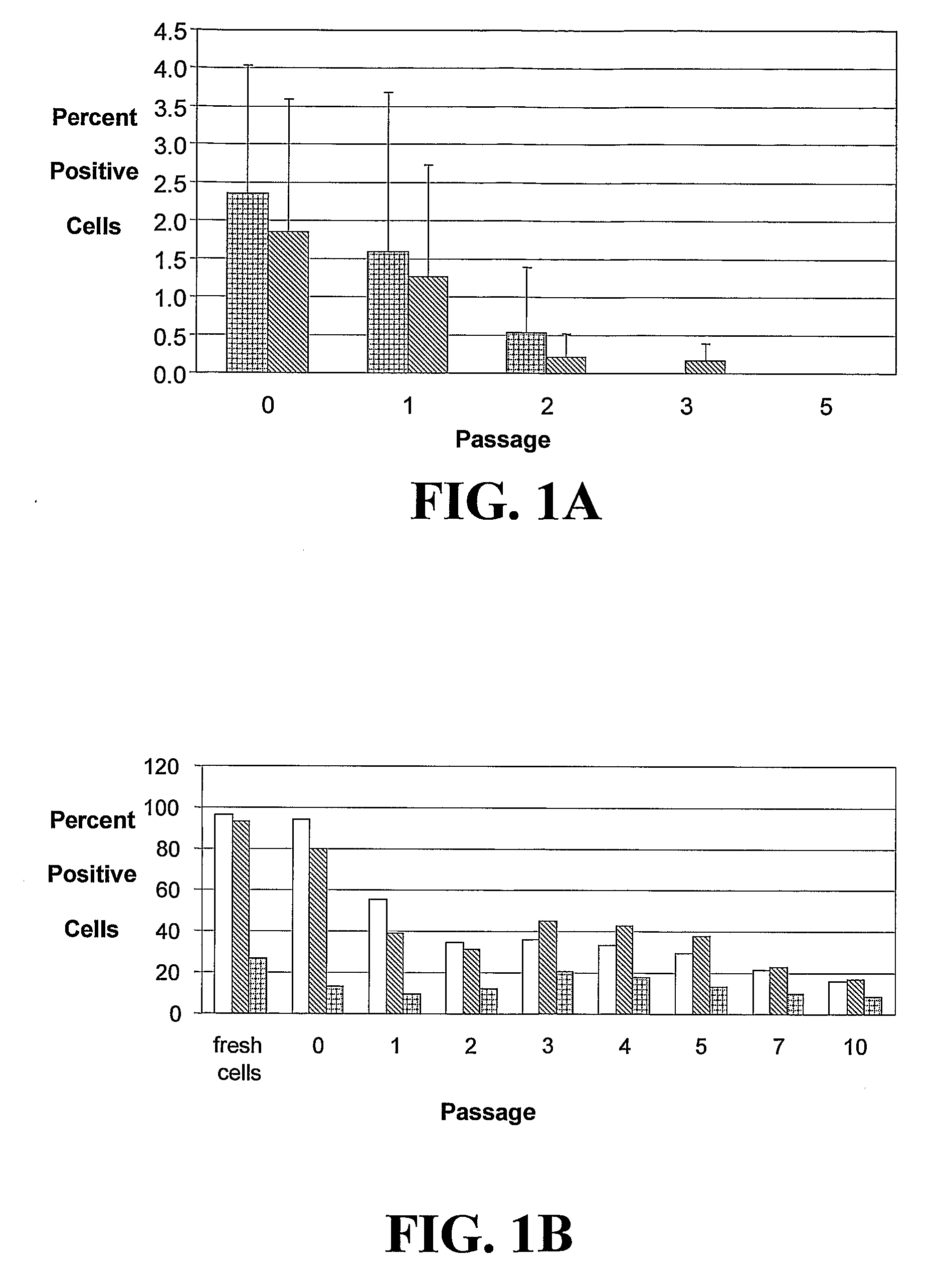
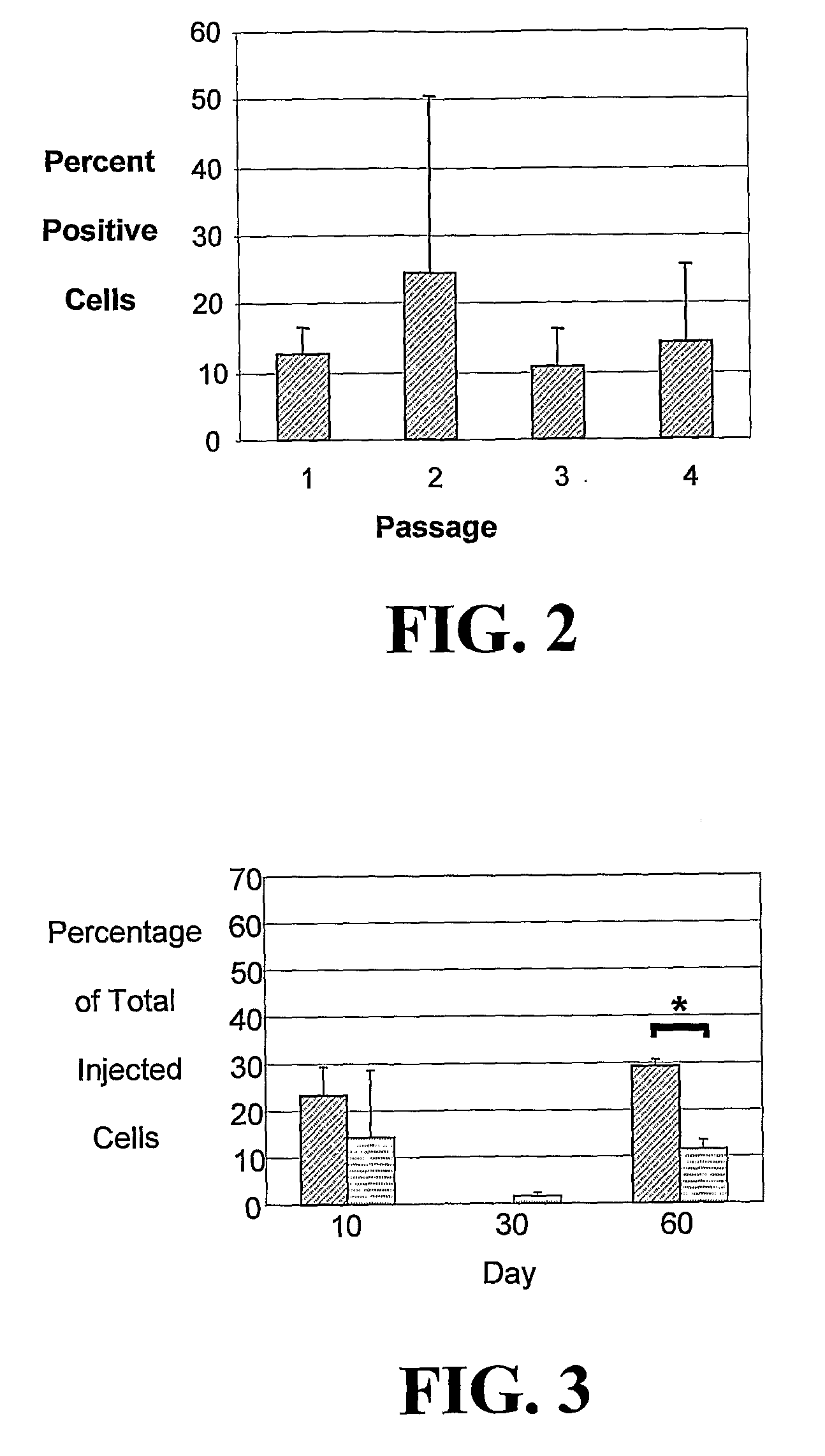
[0222]The studies described herein investigate, at the single cell level, the potential of hASCs to contribute to microvascular growth when injected in vivo, and we have found that injected hASCs migrate to the abluminal surface of microvessels and alter their cell morphology to conform to the curvature of the microvessel in a manner that is consistent with perivascular (and not endothelial) cell behavior. Also examined herein is the expression of various perivascular cell markers, focusing on the pericyte population, a cell population defined by their morphological structure around microvessels with cell processes extending beneath the basement membrane. Since pericytes have been suggested to contribute to microvessel growth and maintenance, it was determined whether hASCs function as microvascular support cells by analyzing their perivascular investment in relation to changes in total vascular density. The present application discloses a new role for human adipose-derived stromal ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Density | aaaaa | aaaaa |
| Gene expression profile | aaaaa | aaaaa |
| Morphology | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com