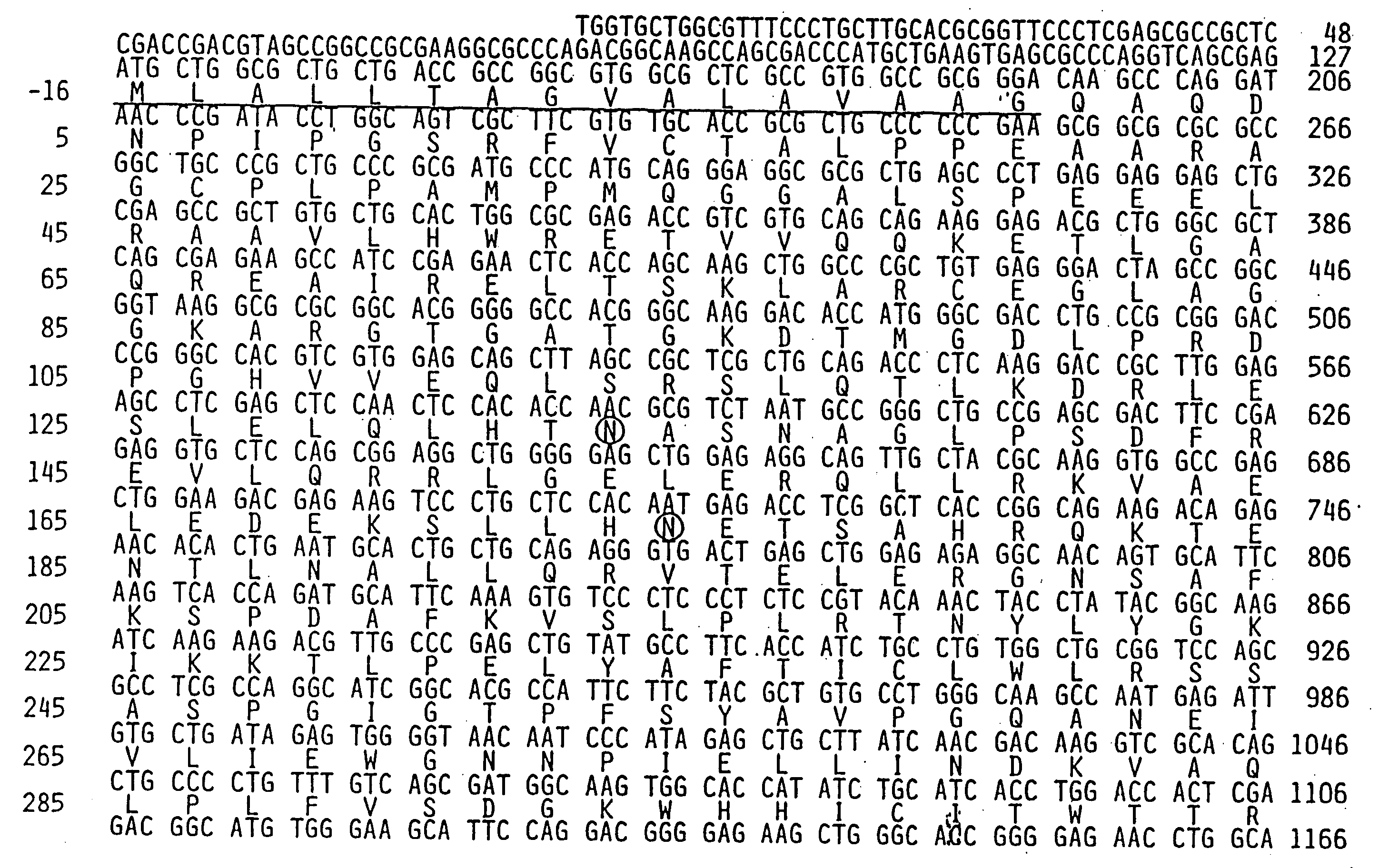
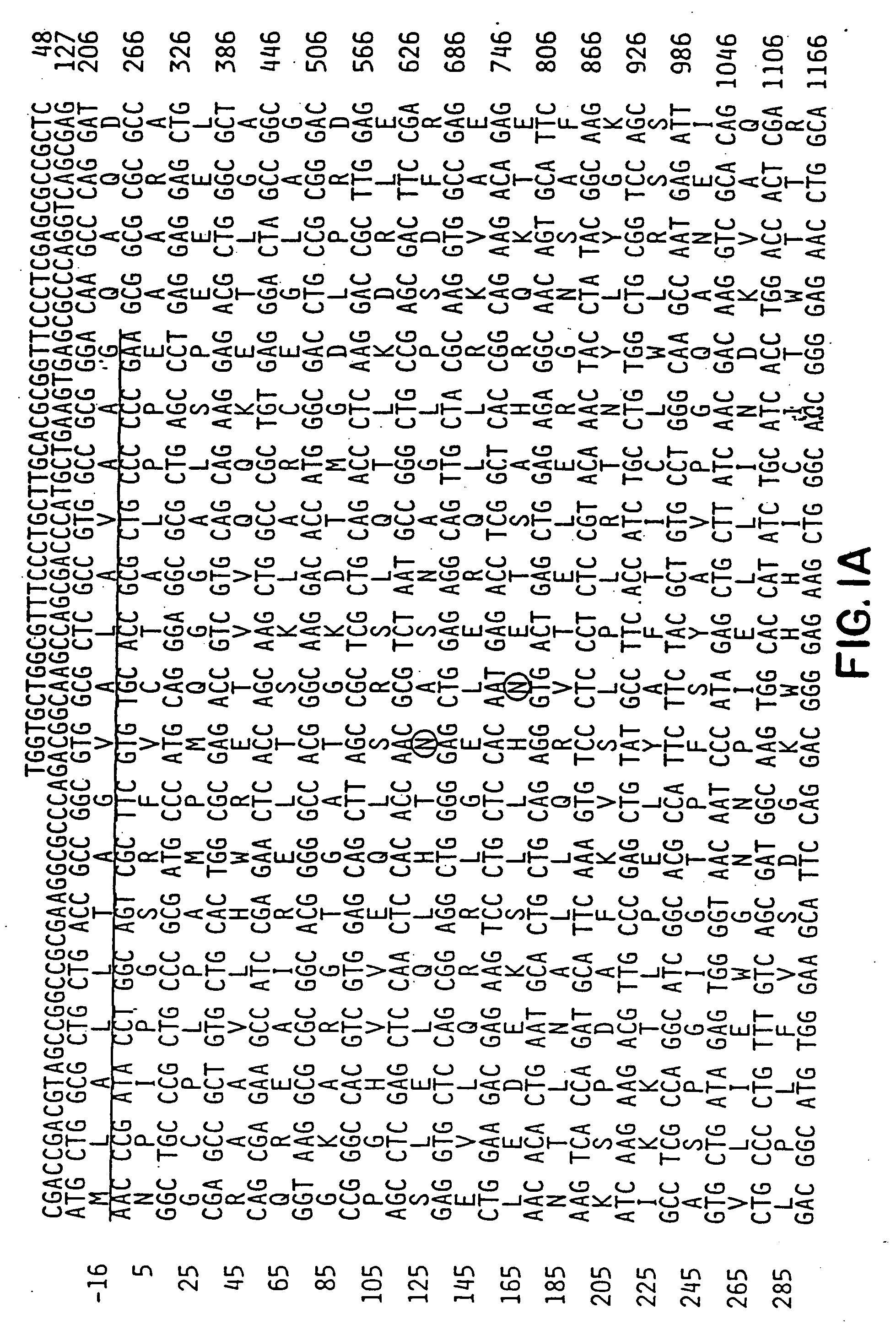
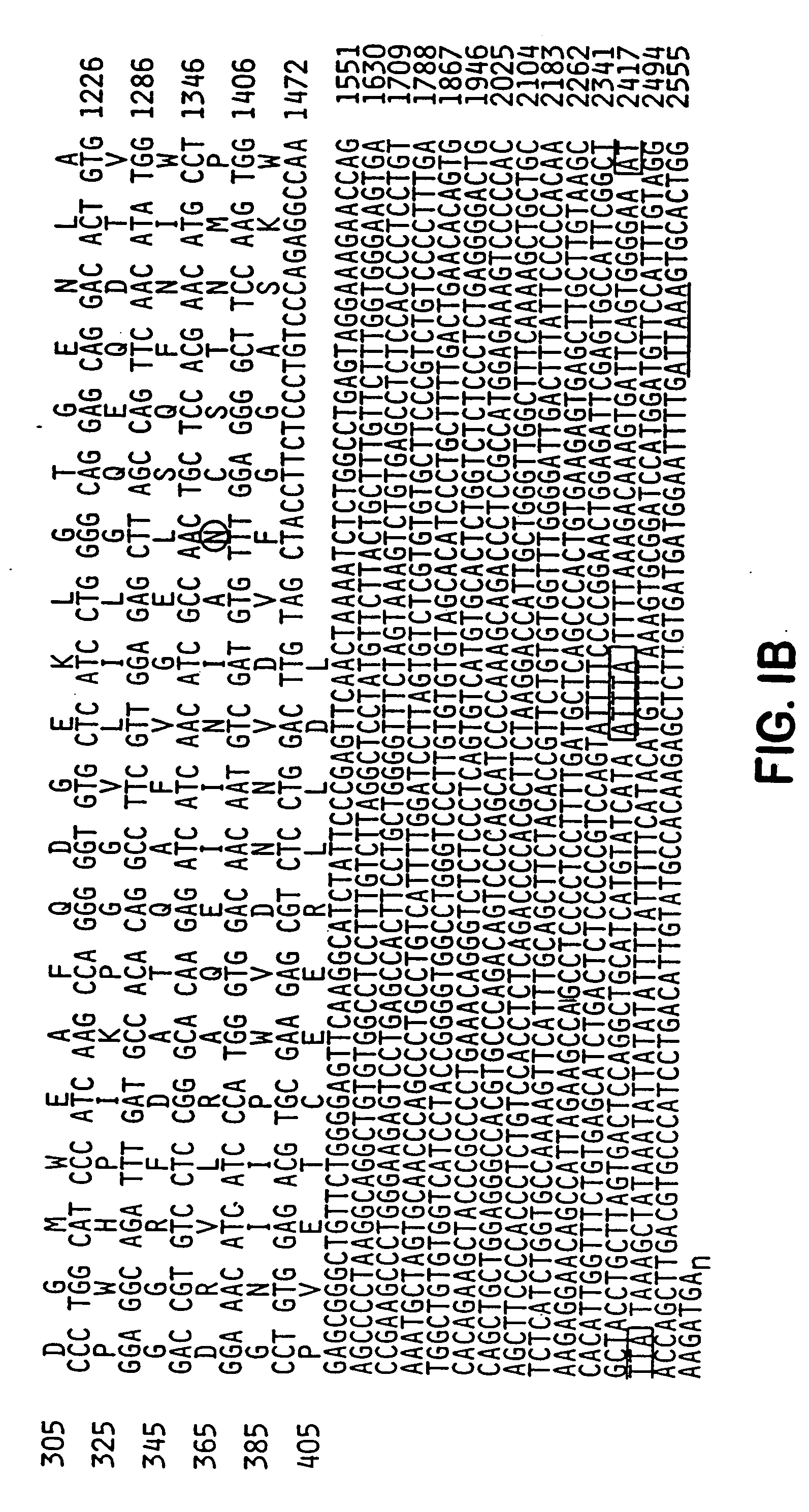
Method for identifying compounds which affect synaptogenesis
a technology of compound and synaptogenesis, applied in the field of molecules involved in synaptogenesis, can solve the problems of additional complexity in the formation of central excitatory synapses, and achieve the effects of increasing the number of excretory synapses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Narp is Enriched at Excitatory Synapses in a Subpopulation of Neurons from Hippocampus and Spinal Cord
[0089]To study Narp protein, a rabbit polyclonal antibody was generated against a full length GST fusion protein of Narp. On western blot, this antibody recognized a single broad protein band centered at 56 kDa in rat brain, similar to the size of recombinant Narp expressed in detergent extracts of transfected HEK-293 cells. Narp protein was also detected as a similar sized triplet in rat testes, but not in other peripheral tissues. This restricted distribution of Narp protein parallels the Narp mRNA expression previously reported (Tsui et al., supra, 1996). The broadness of the Narp band in brain is consistent with the observation that Narp is glycosylated (Tsui et al., supra, 1996). In non-reducing gels, Narp migrates as a multimer, with a size greater than 220 kDa, consistent with the known ability of members of the pentraxin family to covalently multimerize through disulfide bon...
example 2
Transfected Narp Accumulates at Excitatory Synapses
[0096]In order to characterize the synaptic targeting of Narp in neurons, a C-terminal myc-tagged version of Narp (designated myc-Narp) was transfected into cultured spinal neurons (Dong et al., Nature 386:279-84, 1997). After 72 hours of expression, live staining with an anti-myc (mouse) antibody, followed by a FITC labeled anti-mouse secondary, was used to reveal the surface distribution of the transfected Narp. Subsequent staining of the same cells, after fixation and permeabilization, with an AMCA labeled anti-myc (rabbit) antibody was used to identify transfected neurons and their axons and dendrites, while GluR1 and GAD staining were used to identify excitatory and inhibitory synapses, respectively.
[0097]Staining of transfected neurons indicated that myc-Narp, similar to endogenous Narp, was distributed throughout the somato-dendritic domain of the transfected neuron and was also specifically localized to excitatory synapses. ...
example 3
Synaptic Narp is Derived from Both the Presynaptic and Postsynaptic Neuron
[0099]Since only 1 to 3% of the neurons in a given experiment are transfected by the procedure described above, it was relatively easy to distinguish myc-Narp that came from the postsynaptic cell (surface stain associated with the large proximal processes of an isolated transfected cell) from myc-Narp originating from the presynaptic cell (surface stain associated with a solitary thin process coursing over a non-transfected cell body or dendrite which is not in continuity with any visibly transfected cell). This operationally defined distinction between axon and dendrite was verified to be nearly 90% specific in pilot experiments in which labeled processes were stained with anti-Tau antibodies to distinguish axons from dendrites. As discussed above, 74% of excitatory synapses in transfected neurons had clear surface myc-Narp stain, indicating that Narp is targeted to the synapse from the postsynaptic neuron. H...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com