Human complement C3 derivates with cobra venom factor-like function
a technology of cobra venom and complement, which is applied in the field of chimeric derivatives of human complement c3, can solve the problems of significant structural differences between the two molecules, and achieve the effect of avoiding or ameliorating reperfusion injury and increasing the efficiency and/or effectiveness of gene therapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Production of Human Complement C3 / CVF Hybrid Proteins
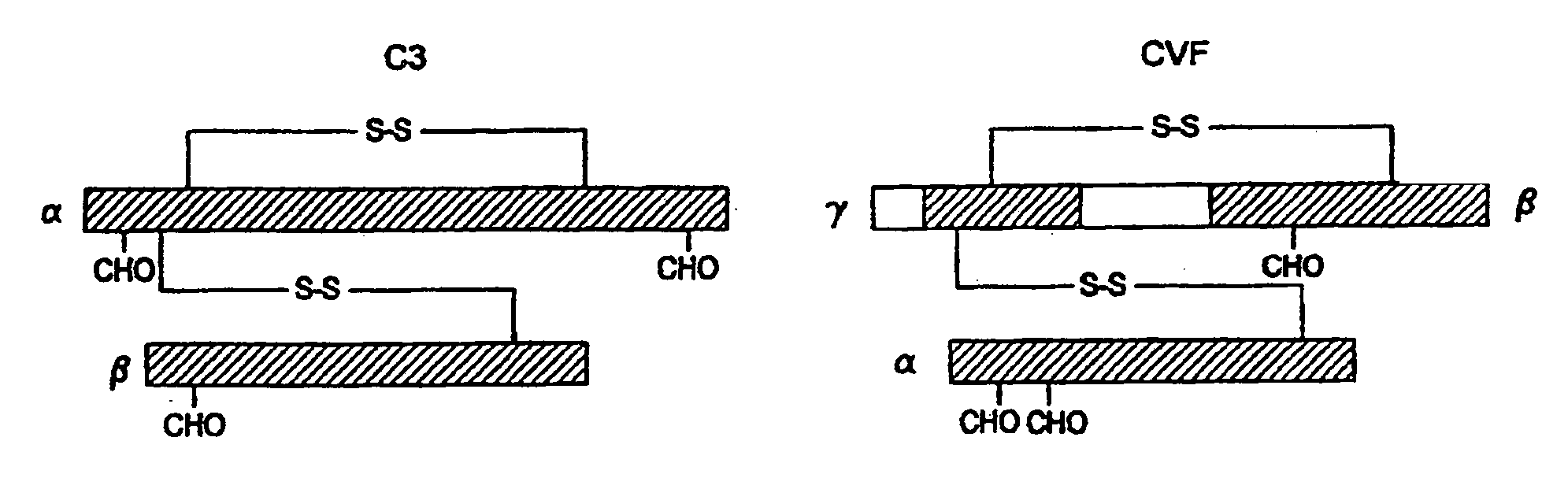
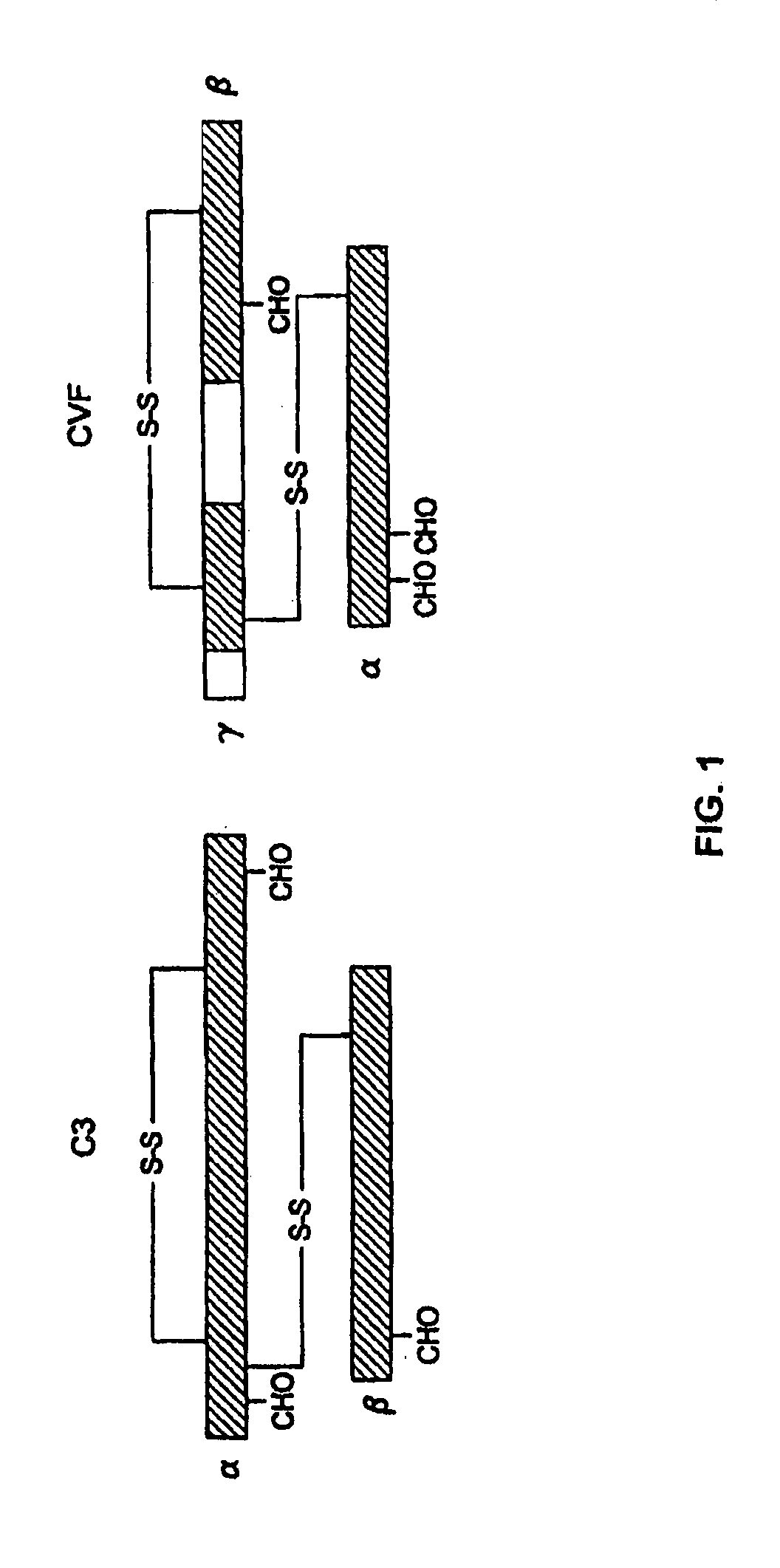
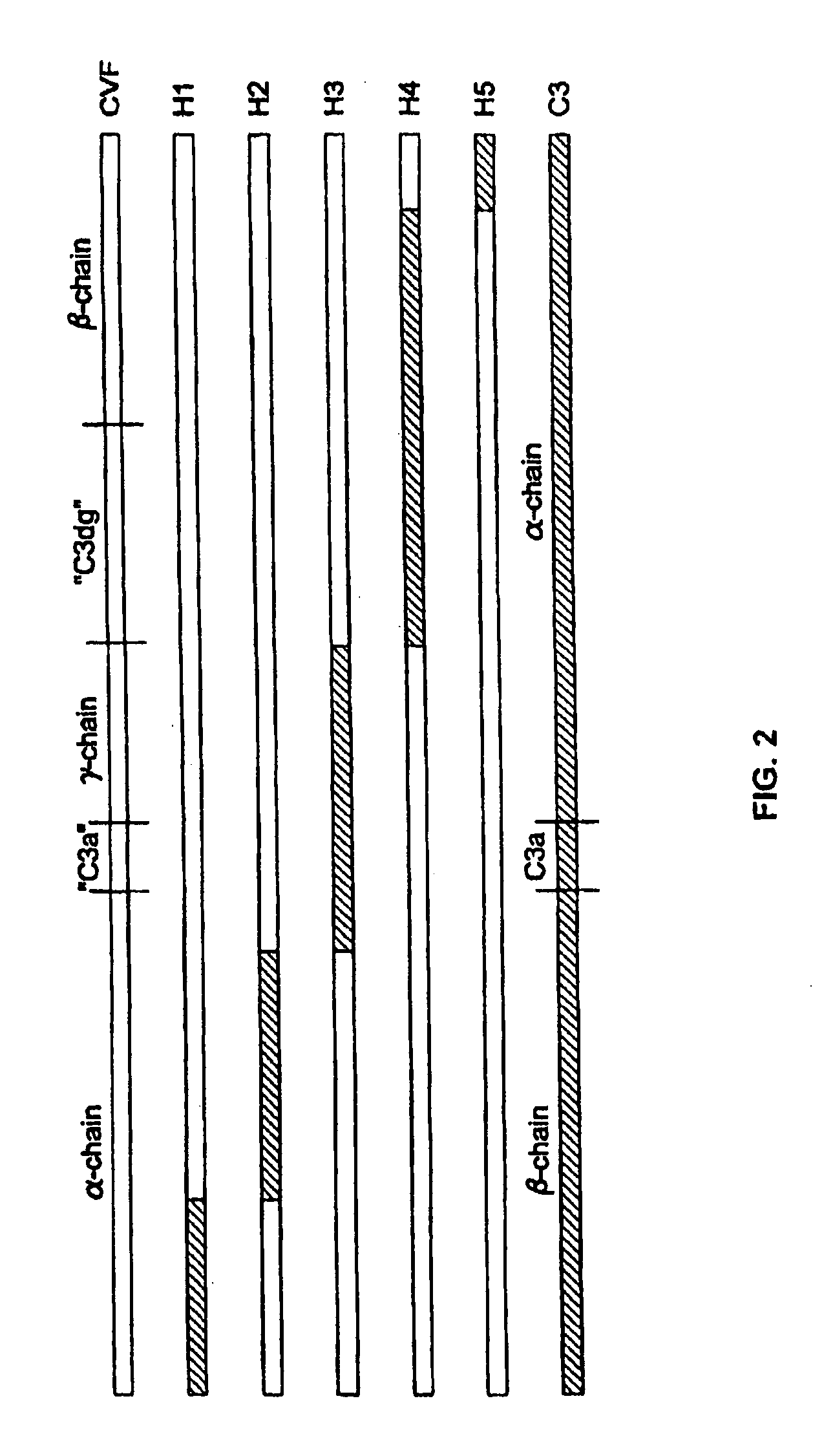
[0076]The replacement of human C3 sequences with CVF sequences representing important structural features for CVF specific functions allows the creation of C3 derivatives with CVF-like functions. Thus, embodiments of the invention are directed to generation of human C3 derivatives that exhibit the CVF-specific function of depleting complement by forming a stable convertase for use as a novel therapeutic agent to deplete complement in clinical situations where complement activation is part of the pathogenesis. Because the structural changes in human C3 caused by CVF-specific sequences are minimal, it is clear that the modified C3 molecules can exhibit significantly reduced or event absent immunogenicity.
[0077]The human C3 molecules in Table 1 are engineered to contain specific CVF sequences so as to create human C3 derivatives with CVF functions. Some embodiments of the invention provide smaller substitutions in these areas, to def...
example 2
Expression of Modified human C3 Proteins
[0087]The proteins were produced in the Drosophila S2 cell system, using the Drosophila Bip signal sequence for secretion of the proteins. Briefly, the plasmids pMB / HC3-1550, pMB / HC3-1504, pMB / HC3-1496, pMB / HC3-1550 / 1617, and pMB / HC3-1348 were transfected into Drosophila S2 cells using the calcium phosphate method of Chen and Okayama (Chen, C., and Okayama, H. (1987) Mol. Cell. Biol. 7(8), 2745-2752, herein incorporated by reference in its entirety). S2 cells were transfected with a mixture of expression plasmid and pCoBlast, using a ratio of 19:1 (w:w). Following transfection, cells containing both plasmids were selected using blasticidin (25 μg / ml). For expression, 1-liter cultures of transfected cells were grown in serum-free medium (Hi-Five plus L-glutamine), in the absence of blasticidin. When the cells reached a density of 5×106 cells / ml., production of the recombinant proteins was induced by the addition of CuSO4 to a final concentratio...
example 3
Results of the Activity Measurements of the Modified Human Complement C3 Proteins
[0090]The purified modified human C3 protein hybrids were subjected to a number of functional analyses as follows.
[0091]Complement Depletion:
[0092]This assay measures the ability of a protein to deplete complement in human (or other) serum. The assay was done in two steps. In the first step, the protein of interest was diluted to the desired concentrations in buffer, usually by serial dilution (typically from less than a nanogram / microliter up to approximately 320 ng / microliter or 3.2 μg in the 10 microliters used in the assay). Then, a 10 μl aliquot of the diluted protein was mixed with undiluted serum. The mixture was incubated at 37° C. for 3 hours, which allows the protein to activate complement by forming a C3 convertase. The convertases formed were then able to activate C3 in the serum. Then, to measure the amount of complement activity left, the serum was diluted and mixed with antibody-sensitize...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com