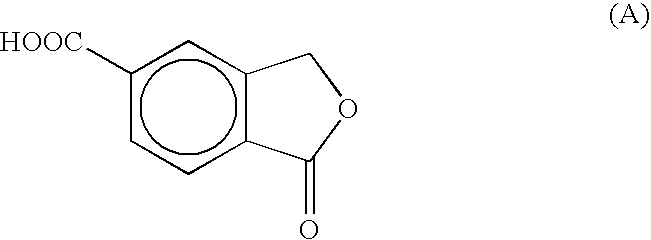
Process for the preparation of 5-Carboxyphthalide
a technology of carboxyphthalide and process, which is applied in the field of process for the preparation of isobenzofuran derivatives, can solve the problems of difficult recovery of end products, inconvenient industrial scale-up, and thick reaction mass, and achieves high purity, easy control, and good yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
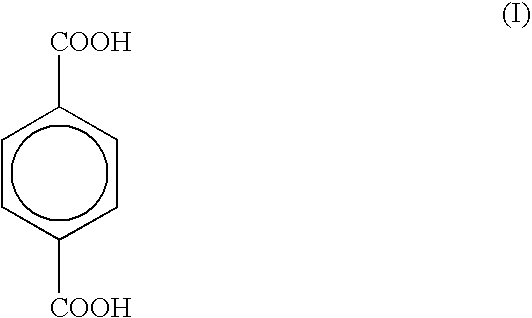
[0020]To 800 ml of fuming sulfuric acid, containing about 27% of SO3, 260 g (1.56 m) of terephthalic acid are added, under stirring, in 15 minutes and without exceeding the temperature of 25° C. To the thick suspension thus obtained, 120 g (1.33 m) of 1,3,5-trioxane are added under stirring without exceeding the temperature of 35° C., then stirring is continued for 20-30 minutes without cooling, whereby the temperature of the mixture rises to 45-50° C. The mixture is heated to 120° C. and it is noted that, already at 90° C., the mass becomes clear whilst at 120° C. a light exothermia is observed which brings the temperature to 135-140° C. The mixture is kept 6 hour under stirring at this temperature, then it is cooled to 20° C. and poured in 3000 g of crushed ice without exceeding the temperature of 25° C. To the mixture thus obtained, a 15% w / w solution of sodium hydroxide is added to a pH≅6 (about 6500-7000 ml thereof are needed), by keeping the temperature at 35-40° C. by water-c...
example 2
[0021]To 800 ml of fuming sulfuric acid, containing about 27% of SO3, 260 q (1.56 m) of terephthalic acid are added, under stirring, in 15 minutes without exceeding the temperature of 25° C. By maintaining the stirring, 60 g (0.665 m) of 1,3,5-trioxane are added portionwise to the thick suspension thus obtained, whereby the temperature rises to about 25° C. The mixture is cooled to 10-15° C. in 30 minutes, then a further 60 g (0.665 m) of 1,3,5-trioxane is added thereinto. The mixture is heated and it is observed that at 90° C. the mass becomes clear. The temperature is brought to 120° C. and the mixture is kept 10-15 minutes under these conditions, whereby the temperature may rise to 135-140° C. If no exothermia is observed, the mixture is nevertheless heated to 130-135° C. and kept 4 hours under these conditions. The cooled mixture is poured, in about 1 hour and without exceeding the temperature of 25-35° C., into 3000 g of crushed ice. To the mixture thus obtained, 8000-8500 ml o...
example 3
[0022]To 153 ml of fuming sulfuric acid, containing about 27% of SO3, 50 g (0.3 m) of terephthalic acid are added, under stirring, at room temperature, then 23 g (0.25 m) of 1,3,5-trioxane are added thereinto in two portions, by cooling to a temperature of 15-18° C. after any addition. At the end of the addition, the mixture is left 30 minutes under stirring at room temperature, then it is heated at 135-145° C. and let under stirring for 2-2.5 hours at this temperature until the end of the reaction. The reaction mixture is cooled to a temperature lower than 3° C., then 100 ml of glacial acetic are added thereto by keeping the temperature at about 25° C. At the end of the addition, the mixture is let to stand 60 minutes under stirring at 20-25° C. and filtered. The wet product is suspended in 1900 ml of water. The suspension is heated up to 25-30° C. under stirring, its pH is adjusted to about 8 by gradual addition of 175 g of sodium bicarbonate. The solid is filtered off on Celite® ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com