Medical devices having antimicrobial coatings thereon
a technology of antimicrobial coating and medical devices, which is applied in the direction of intraocular lens, packaging goods type, peptide/protein ingredients, etc., can solve the problems of affecting the use of the eye, affecting the ocular health of the eye in which the lens is located, and not possessing all the properties desired for polymeric compositions having antimicrobial properties, etc., to achieve low cytotoxicity and high antimicrobial
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Coating Solutions
Polyacrylic Acid (PAA) Solution
[0095]A solution of polyacrylic acid (PAA) having an averaged molecular weight of about 90,000 is prepared by dissolving a suitable amount of PAA in water to have [PAA]=0.001 M. PAA concentration is calculated based on the repeating unit in PAA. Once dissolved, the pH of the PAA solution is adjusted to a desired value.
Poly(allylamine hydrochloride) (PAH) Solution
[0096]A solution of poly(allylamine hydrochloride) (PAH) having an averaged molecular weight of about 60,000 is prepared by dissolving a suitable amount of the material in water to form a 0.001 M PAH solution. PAH concentration is calculated based on the repeating unit in PAH. Once dissolved, the pH of the PAH solution is adjusted to a desired value.
Polyquat (PQ) Solutions
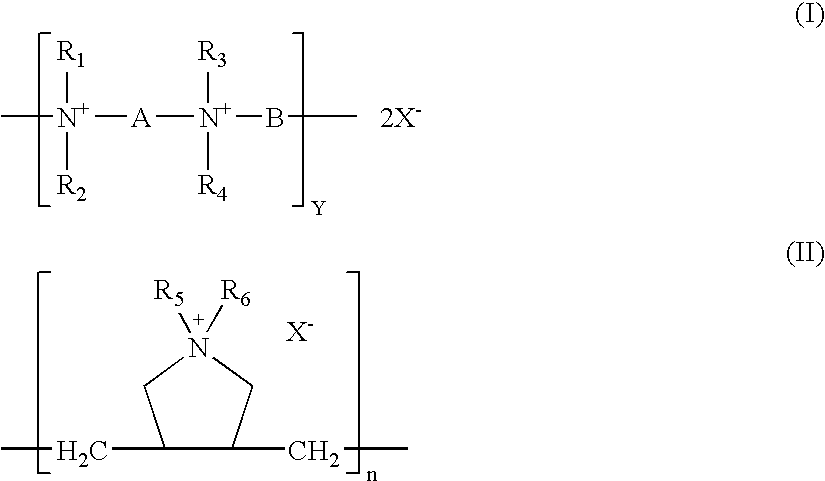
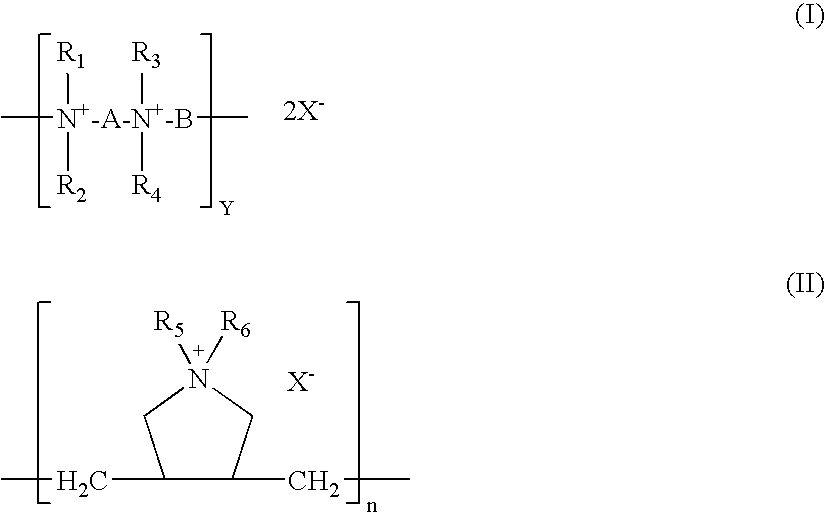
[0097]A solution of polyquat (PQ6-6) of formula (I), in which R1, R2, R3, and R4 are methyl groups, and A and B are hexamethylene groups, is prepared by dissolving a suitable amount of PQ6-6 in ...
example 2
Preparation of Contact Lenses Having LbL Coatings
[0102]This example illustrates LbL coatings and several types of antimicrobial coatings which are formed on soft contact lenses made of a fluorosiloxane hydrogel material, lotrafilcon A.
(1) Coating comprising four and half bilayers:
[0103]PAA / PAH / PAA / PAH / PAA / PAH / PAA / PAH / PAA
[0104]The contact lenses are dipped in a PAA solution (0.001 M, pH2.5) for 30 min, rinsed with ultra-pure water, then dipped in a PAH solution (0.001 M, pH7.5) for 5 minutes, rinsed with ultra-pure water for 1 minute. Three more bilayers are added by alternatively dipping in the solutions of PAA (0.001 M, pH 3.5) and PAH (0.002M, pH 7.5), with a rinse step in-between. The contact lenses with four bilayers of polyelectrolytes is dipped in the PAA solution (0.001 M, pH 3.5) and rinsed with ultra-pure water for 1 minute. The lenses are then packaged in saline and sterilized.
(2) Antimicrobial Coating comprising 6 Bilayers:
[0105]PAA / PAH / PAA / PAH / PAA / PAH / PAA / PAH / PA / PAH / PAA / ...
example 3
[0118]This example illustrates how to produce a covalently-attached antimicrobial coating on a contact lens made of lotrafilcon A, lotrafilcon B, or Balafilcon.
[0119]The contact lens is functionalized by spraying with or dipped into a diaziridine compound and then covalently coupling the diaziridine compound to the contact lens via a thermal process. Such functionalised lens is placed in an open dish containing a polyquat (PQ6-6, PQ6-10, PQ6-12, or PDADMAC) solution of about 10 μg / ml and irradiated with blue light for 30 minutes.
PUM
| Property | Measurement | Unit |
|---|---|---|
| contact angle | aaaaa | aaaaa |
| molecular weights | aaaaa | aaaaa |
| molar charge ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com