Conversion of urea to reactants for NOx reduction
a technology of reactants and urea, applied in the field of system for nox reduction, can solve the problems of releasing pollutants, limiting its use in some locations, and ammonia typically used in scr and sncr presents problems of its own
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0034]Reference will now be made in detail to the present preferred embodiments of the invention, examples of which are illustrated in the accompanying drawings. The method and corresponding steps of the invention will be described in conjunction with the detailed description of the system.
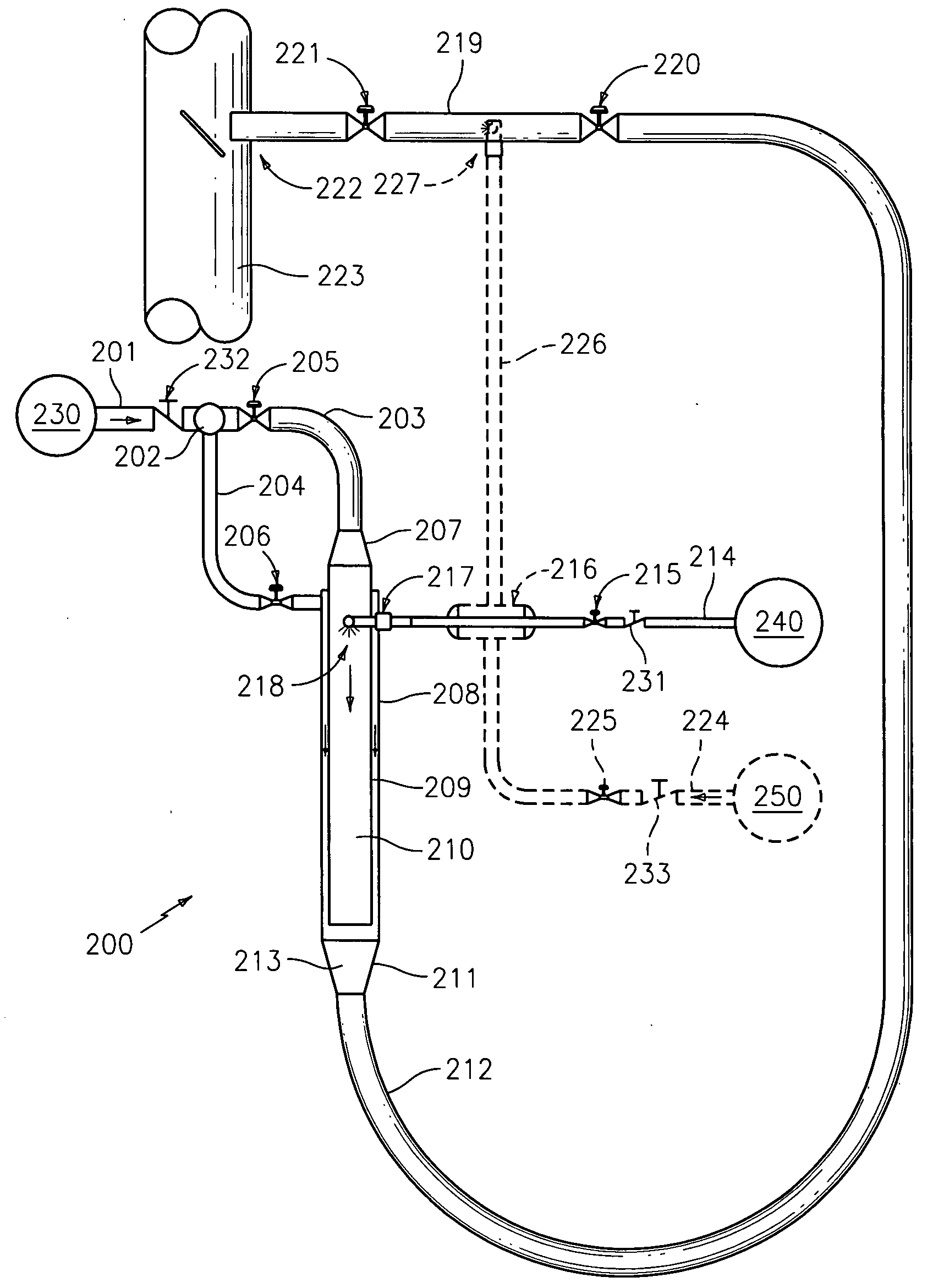
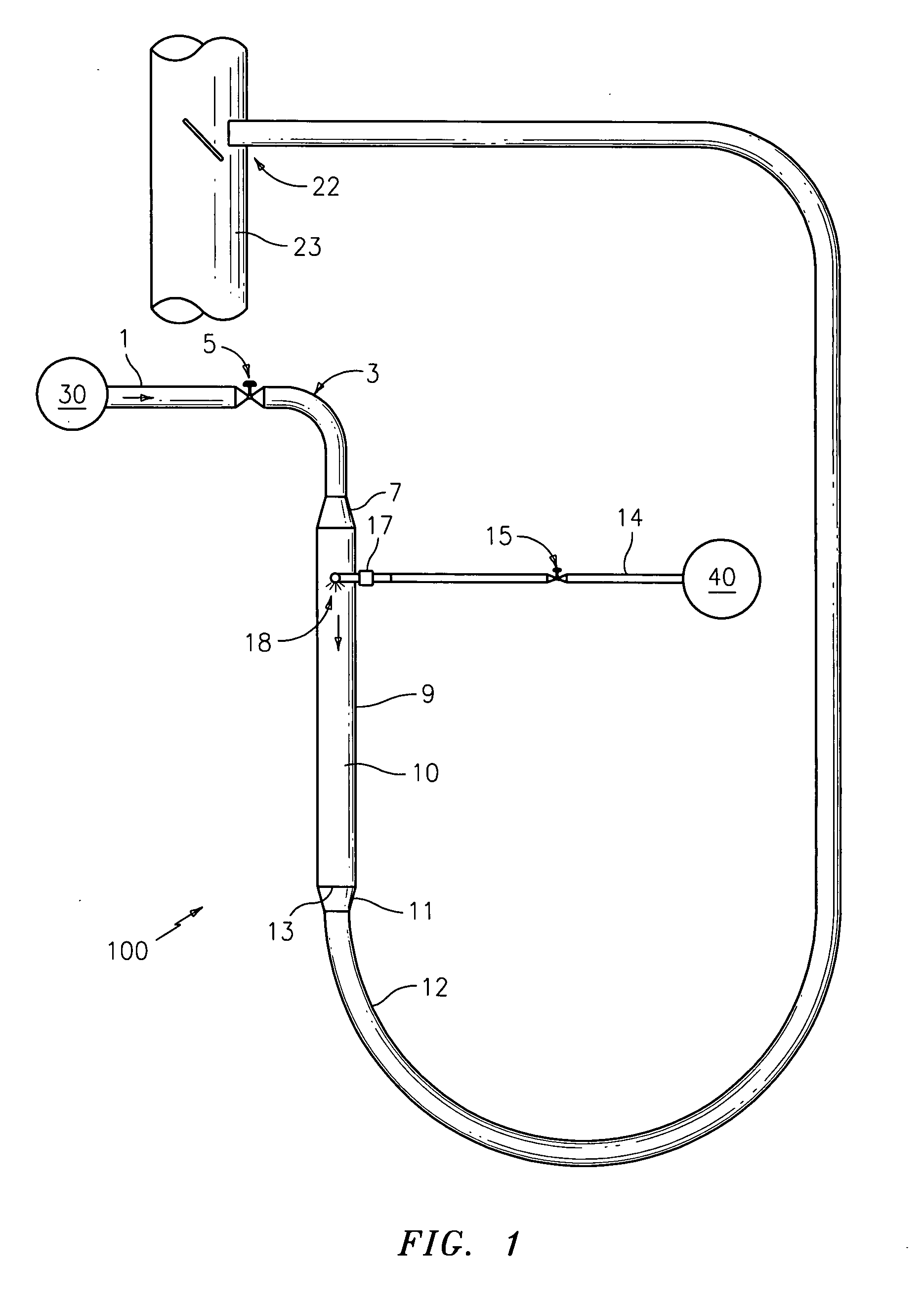
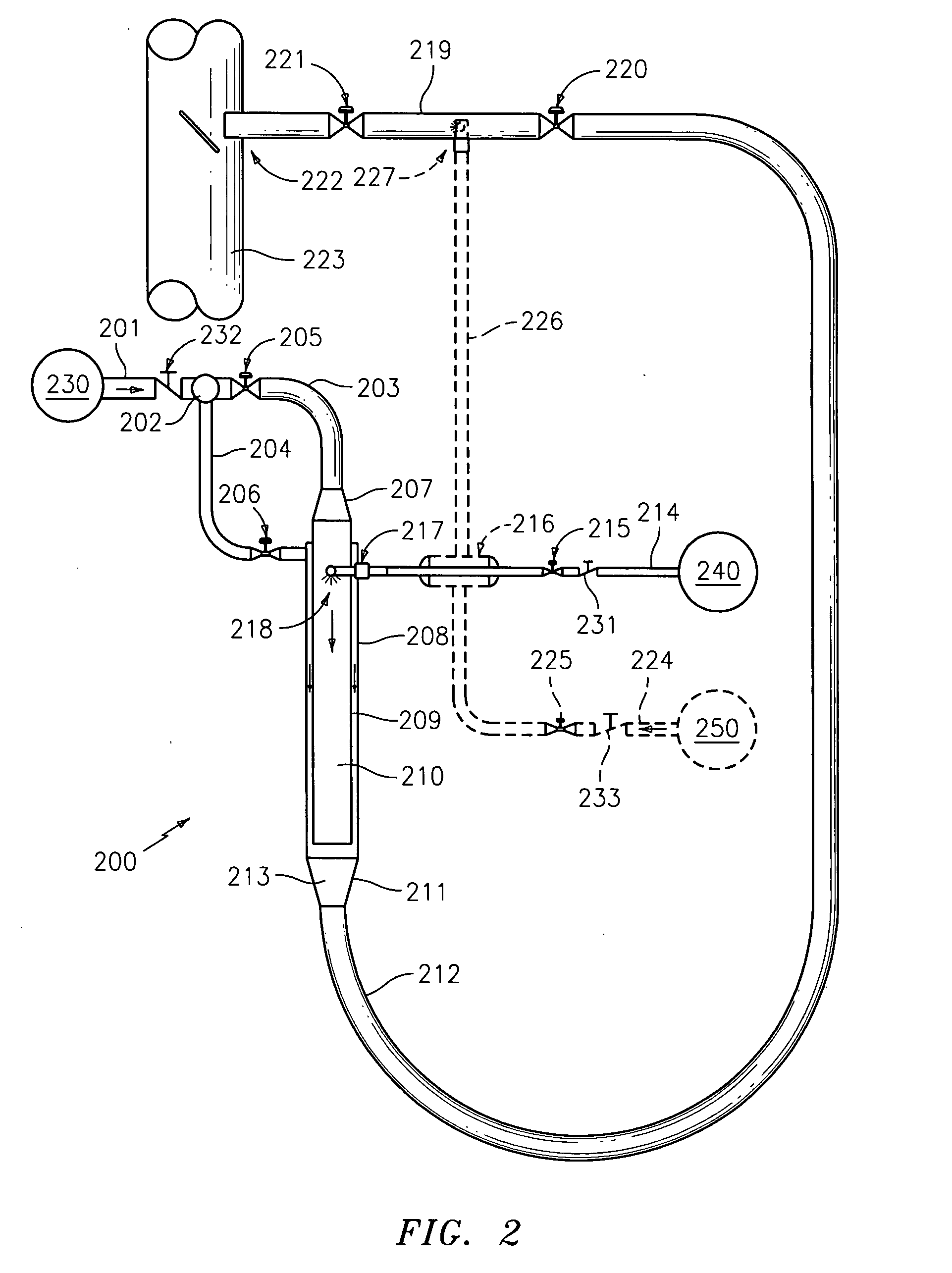
[0035]The devices and methods presented herein may be used for converting urea into reactants such as ammonia that are suitable for use in NOX reduction processes. The present invention is particularly suited for converting urea to ammonia and other reactants for use in processes such as SCR and SNCR.
[0036]In accordance with the invention, a system for converting urea into reactants for removing NOX from industrial emissions is provided including a urea inlet, a steam inlet, and a reactor in fluid communication with the urea inlet and the steam inlet. The reactor is configured and adapted to inject urea from the urea inlet into a steam flow from the steam inlet to convert the urea into at least on...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com