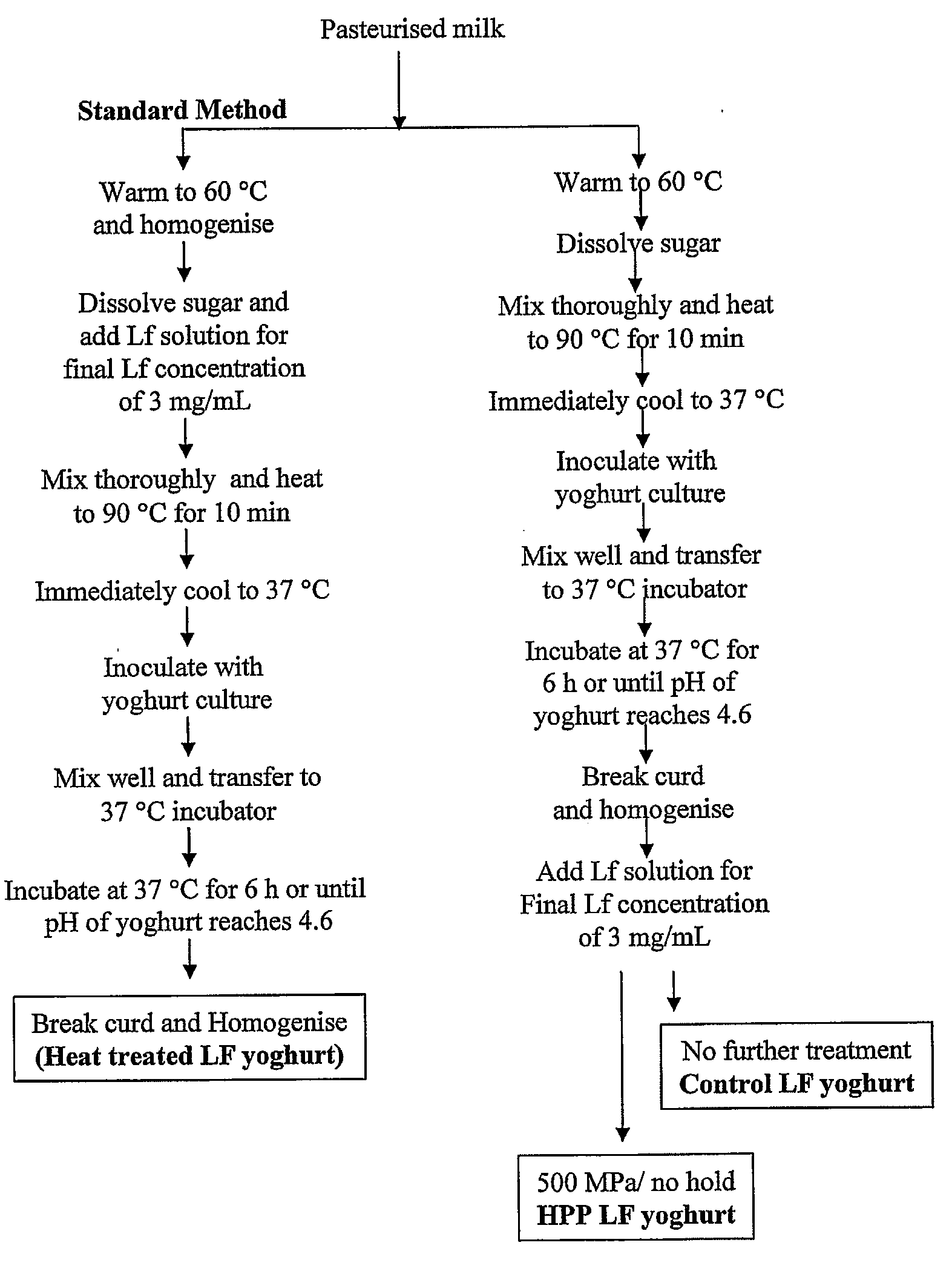
High Pressure Processing of Bioactive Compositions
a bioactive composition and high pressure technology, applied in the direction of transferrins, immune disorders, extracellular fluid disorders, etc., can solve the problems of thermal processing not generally suitable for the production of commercially sterile bioactive products, the like, and affecting the bioactivity of food products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Solution Containing Colostrum Ingredient
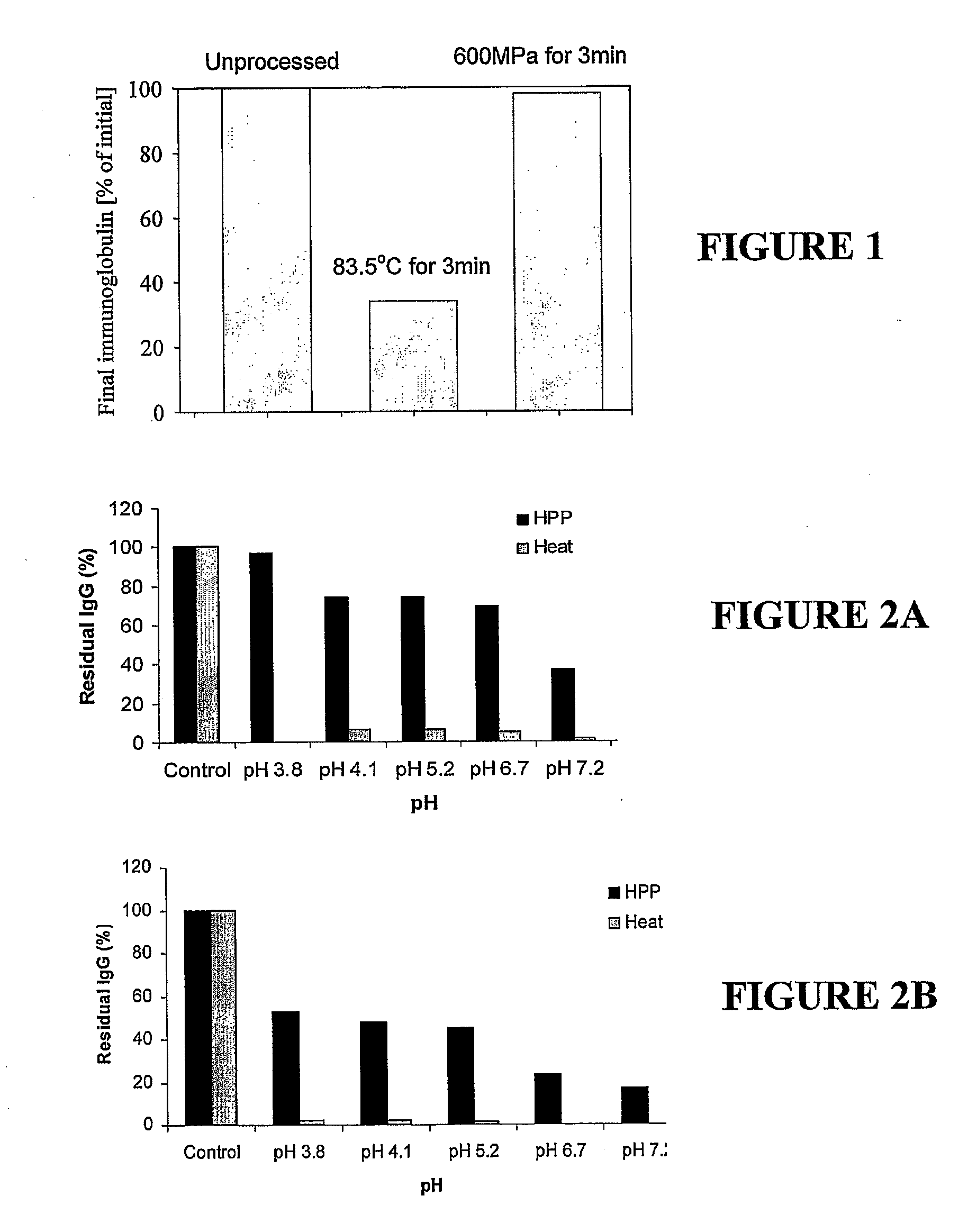
[0205]A colostrum milk protein concentrate powder (Fonterra Co-operative Group Limited) containing 80% protein (and 6.6% immunoglobulins) was made up with water and the pH adjusted to 3.5 with lactic acid to yield a 3.6% protein solution. A sample of the solution was then heat-treated or pressure-treated at 83.5° C. or 600 MPa respectively, held for 3 minutes, and the quantity of immunoglobulins in the treated beverages was measured by HPLC and compared to the quantity in the unprocessed solution. The results are shown in FIG. 1. An HPP unit from Stansted Fluid Power Ltd, UK was used for all examples.
example 2
Solutions Containing Colostrum MPC Ingredient
[0206]Colostrum MPC was dissolved in a 0.3% w / v pectin solution to prepare a 6% w / v stock solution of colostrum MPC. Samples of the colostrum stock solution were acidified to a range of pH values and either heat-processed at 85° C. and held for 10 min or pressure-processed as described below. The residual immunoglobulin G (IgG) of each heat- and pressure-processed sample relative to an unprocessed control at the same pH was measured by HPLC-MC. The solutions were also challenged with coliforms, yeast and mould and were enumerated for these organisms following pressure treatment at 600 MPa.
[0207]At pH 3.3, a colostrum sample pressure-processed at 400 MPa and held for 3 min had 67% IgG remaining, compared to 2% for heat-processed colostrum.
[0208]At pH 4.1, a colostrum sample pressure-processed at 600 MPa and held for 3 min had 37% IgG remaining, compared to 2% for heat-processed colostrum.
[0209]At pH 3.5, a colostrum sample pressure-process...
example 3
Colostrum MPC Ingredient at Various pH
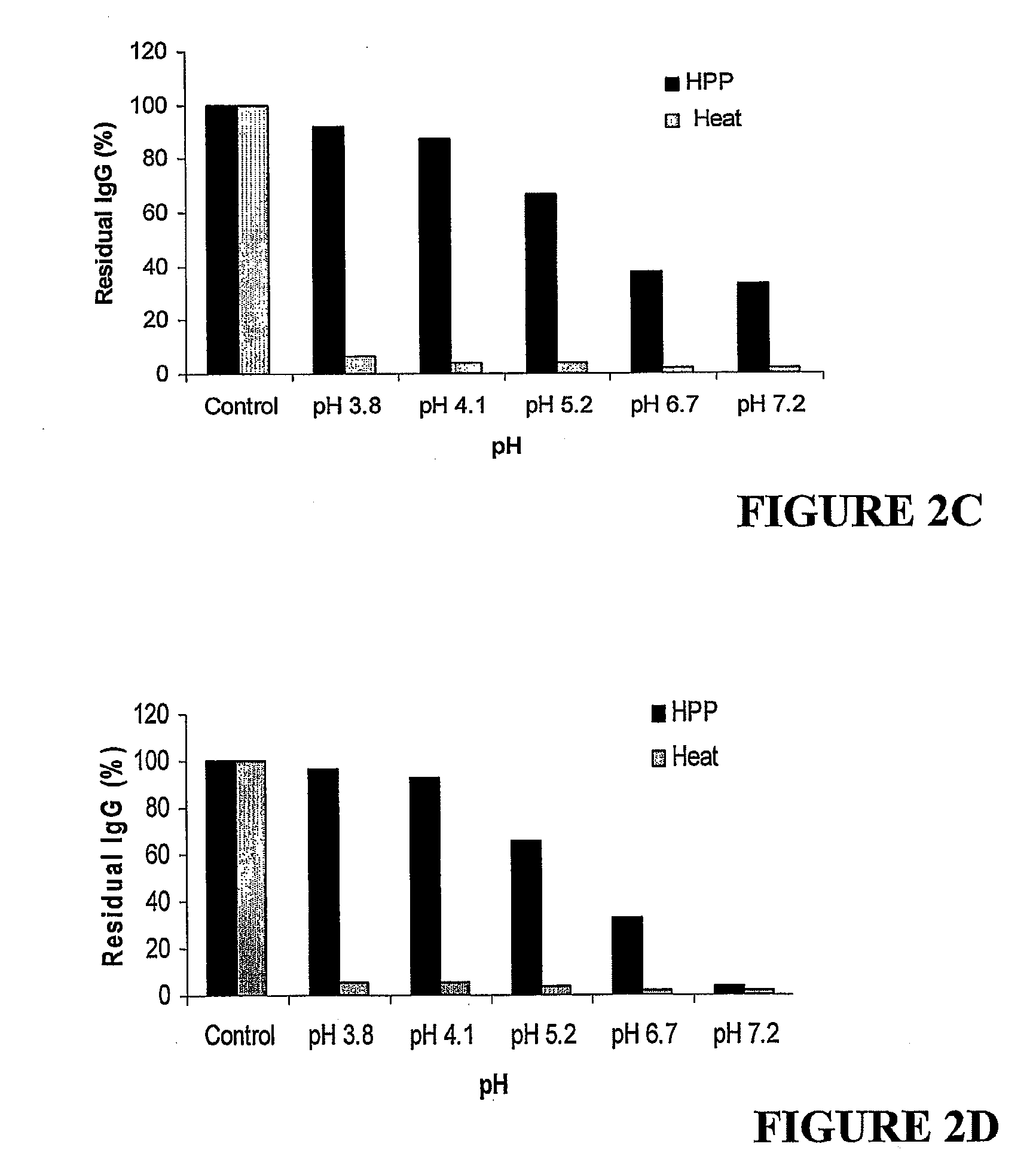
[0213]Colostrum samples from the stock solution of Example 2 were acidified to pH 3.5 to 4.1 and pressure processed at 400 to 600 MPa (no hold) and compared to a heat-processed preparation. In all cases approximately 2% residual IgG was retained after heat treatment, compared to 45-100% residual IgG retained after pressure treatment, with the highest residual IgG at the higher pH. The results are summarised in Table 2.
TABLE 2Residual IgG (%) of heat-or pressure-processed6% w / v colostrum MPCPressurepH 3.5pH 3.8pH 4.1Unprocessed10010010085° C. / 10 min222400 MPa / no hold7592100500 MPa / no hold547391600 MPa / no hold455648
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com