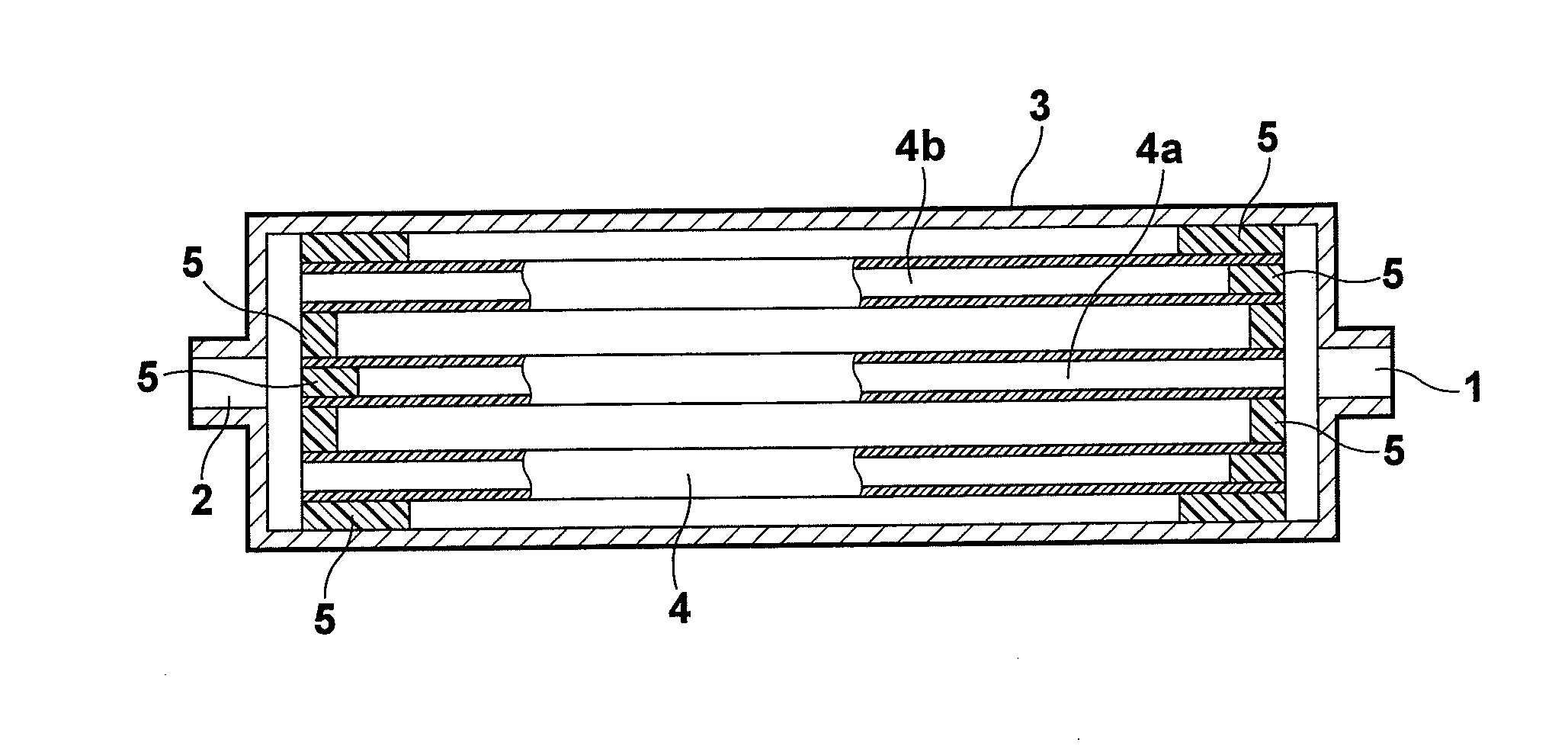
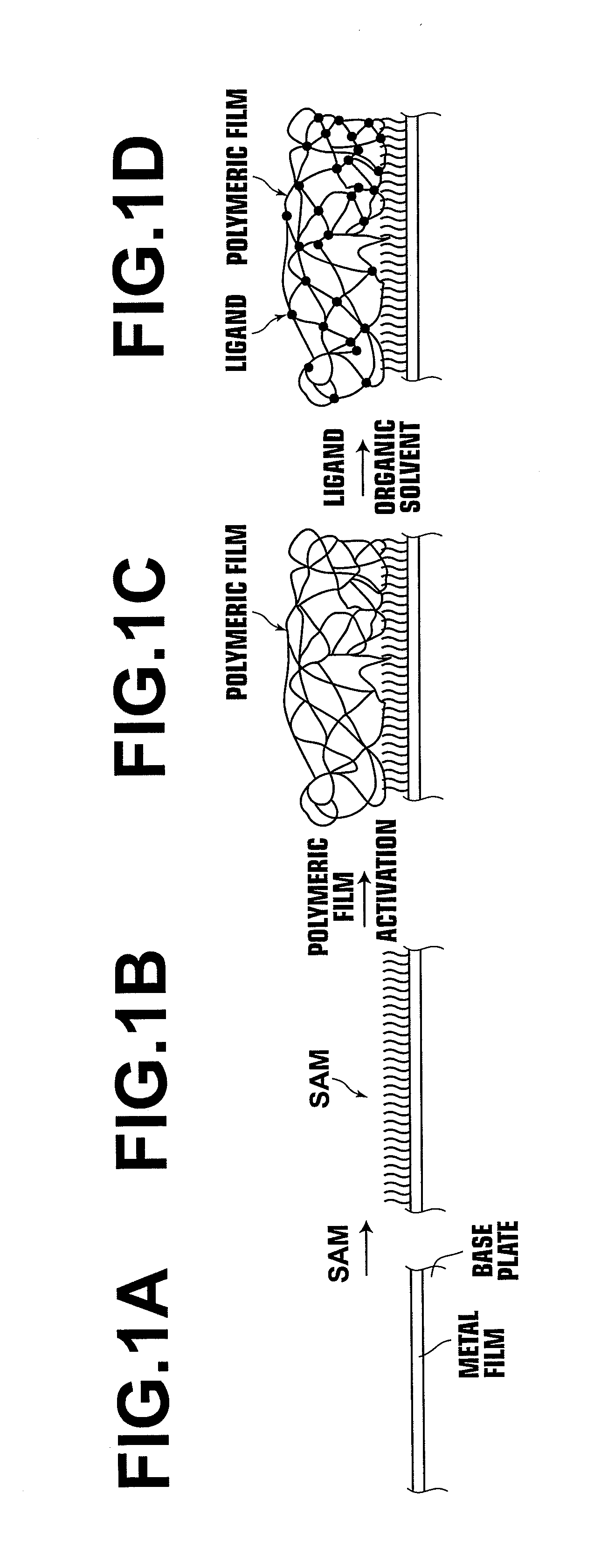
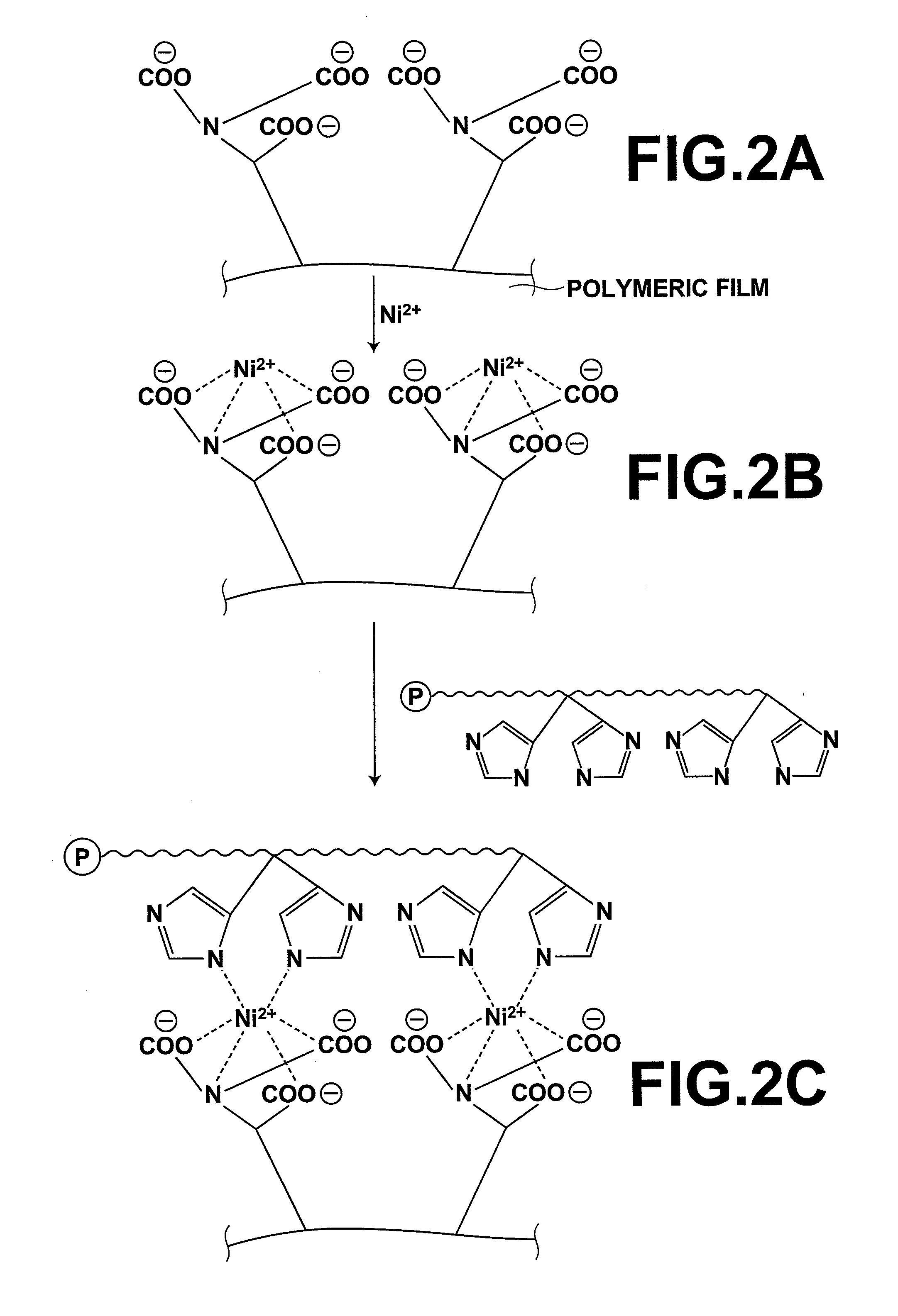
Carrier, process for producing same, bioreactor, and chip for surface plasmon resonance analysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
(Preparation of SAM)
[0196]A chromium film having a thickness of 3 nm and a gold film having a thickness of 20 nm were formed on a polystyrene microwell plate (96Well Microwell Plate, supplied by Nunc) by use of a sputtering technique. Thereafter, a solution, which contained 10 μmol of 6-aminohexanethiol (supplied by Aldrich) dissolved in 8 ml of ethanol and 2 ml of ultra pure water, was allowed to undergo reaction with the gold film, which had been formed with the sputtering technique described above, at a temperature of 40° C. for one hour. The resulting SAM was then washed one time with ethanol and was thereafter washed one time with ultra pure water.
(Activating Esterification of CMD)
[0197]A CMD solution was prepared with processing wherein CMD (molecular weight: 1000,000, supplied by Meito Sangyo Co., Ltd.) was dissolved in ultra pure water so as to have a concentration of 0.5% by weight. Thereafter, a mixed solution, which contained 0.4M of EDC (i.e., 1-(3-dimethylaminopropyl)-3...
example 2
[0201]A carrier was prepared in the same manner as that in Example 1, except that, at the time of the binding of AB-NTA, a solution was prepared by the addition of 0.2 mmol of EDC and 0.04 mmol of NHS to 1 ml of DMSO, 50 μl of the thus prepared solution was added onto the CMD film, and the solution was then allowed to undergo the reaction at the room temperature for 30 minutes.
examples 3 , 4
Examples 3, 4, and 5
[0202]A carrier was prepared in the same manner as that in Example 1, except that the kind of the metal source was changed as listed in Table 1 below.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Polarity | aaaaa | aaaaa |
| Solubility (mass) | aaaaa | aaaaa |
| Hydrophilicity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com