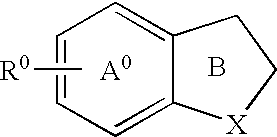
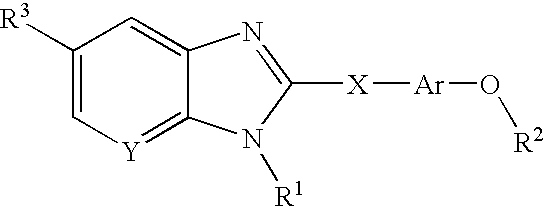
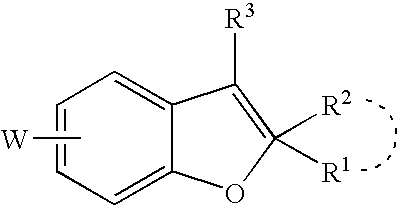
Cannabinoid receptor modulator
a cannabinoid receptor and modulator technology, applied in the field of benzene ringfused 5membered heterocyclic compounds, can solve the problem of limiting the time-window for treatment, and achieve the effect of preventing, treating or diagnosing cerebrovascular diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
reference example 1
Hydroxy(4-isopropylphenyl)acetic acid
[0598]To a mixture of lithium chloride (17.0 g, 418 mmol), potassium hydroxide (44.9 g, 800 mmol) and ice (150 g) was added a solution of bromoform (17.5 mL, 200 mmol) and 4-isopropyl benzaldehyde (30.3 mL, 200 mmol) in 1,4-dioxane (150 mL) at 0° C., and the mixture was stirred at 5-10° C. for 24 hours and then stirred at 35° C. for 24 hours. The aqueous layer was acidified with hydrochloric acid and was extracted with ethyl acetate. The extract was washed with water and then was dried over anhydrous sodium sulfate. The solvent was distilled off under reduced pressure to obtain a residue, which was crystallized from hexane-ethyl acetate to obtain 28.5 g (yield 73%) of the title compound. Melting point: 156-157° C.
[0599]1H-NMR (CDCl3) δ: 1.24 (6H, d, J=7.0 Hz), 2.91 (1H, septet, J=7.0 Hz), 5.21 (1H, s), 7.24 (2H, d, J=8.8 Hz), 7.36 (2H, d, J=8.8 Hz), 2H unidentified.
reference example 2
3-(4-Isopropylphenyl)-4,6,7-trimethyl-1-benzofuran-2(3H)-one
[0600]To a mixture of hydroxy(4-isopropylphenyl)acetic acid synthesized in Reference Example 1 (11.8 g, 60.8 mmol) and 2,3,5-trimethylphenol (12.4 g, 91.2 mmol) was added 70% sulfuric acid (10 mL) at room temperature, and the mixture was stirred at 115° C. for 12 hours. The mixture was added to water and was extracted with diisopropyl ether. The extract was washed with water and a saturated sodium hydrogen carbonate solution, and then was dried over anhydrous sodium sulfate. The solvent was distilled off under reduced pressure to obtain a residue, which was purified by silica gel column chromatography (hexane:ethyl acetate=8:1) to obtain 10.9 g (yield 65%) of the title compound. Melting point: 107-108° C. (hexane-ethyl acetate).
[0601]1H-NMR (CDCl3) δ: 1.22 (6H, d, J=6.6 Hz), 1.93 (3H, s), 2.24 (3H, s), 2.29 (3H, s), 2.88 (1H, septet, J=6.6 Hz), 4.76 (1H, s), 6.76 (1H, s), 7.07 (2H, d, J=8.1 Hz), 7.17 (2H, d, J=8.1 Hz).
reference example 3
3-(4-Isopropylphenyl)-6,7-dimethyl-1-benzofuran-2(3H)-one
[0602]Using hydroxy(4-isopropylphenyl)acetic acid synthesized in Reference Example 1 and 2,3-dimethylphenol, the title compound was synthesized in the same manner as in Reference Example 2. Yield 44%. Melting point: 58-60° C. (methanol).
[0603]1H-NMR (CDCl3) δ: 1.22 (6H, d, J=6.9 Hz), 2.27 (3H, s), 2.32 (3H, s), 2.88 (1H, septet, J=6.6 Hz), 4.85 (1H, s), 6.91 (1H, d, J=7.8 Hz), 6.95 (1H, d, J=7.8 Hz), 7.13 (2H, d, J=8.1 Hz), 7.19 (2H, d, J=8.1 Hz).
PUM
| Property | Measurement | Unit |
|---|---|---|
| cerebral temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com