Methods of inhibiting binding of beta-sheet fibril to rage and consequences thereof
a beta-sheet fibril and rage technology, applied in the field of methods of inhibiting the binding of beta-sheet fibrils to rage and consequences thereof, can solve the problems of inability to form fibrils in clinical dementia and the lik
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
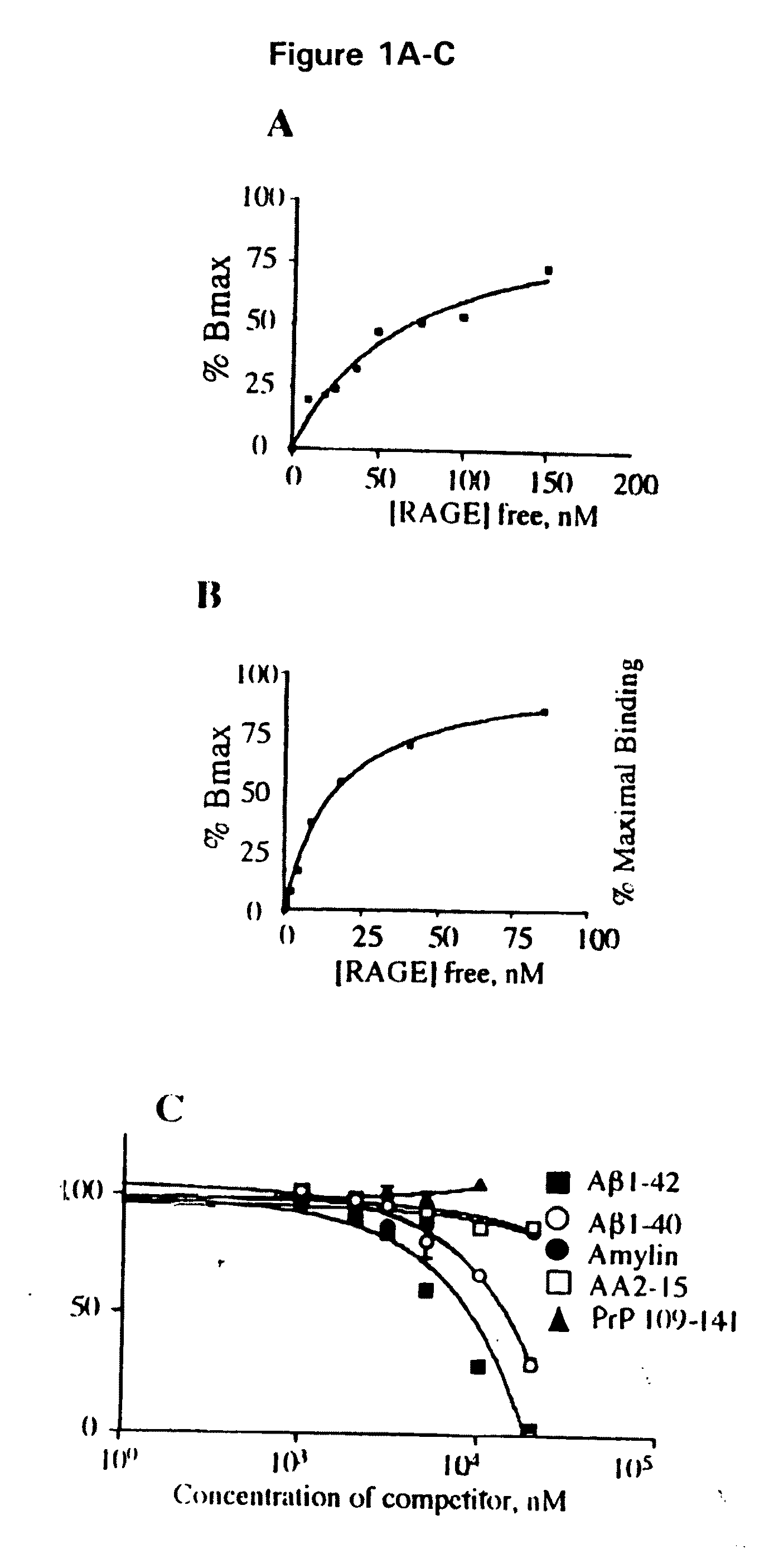
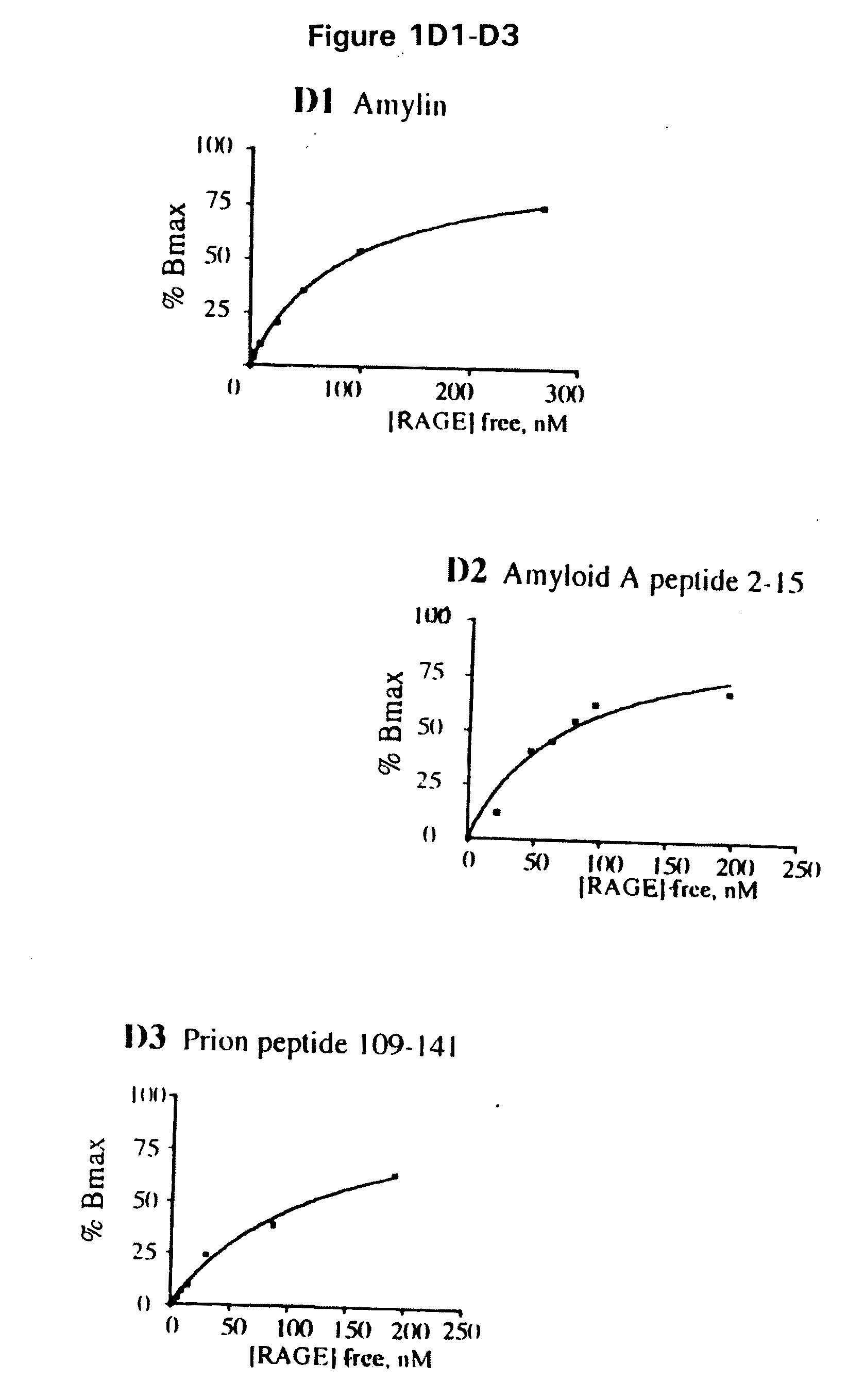
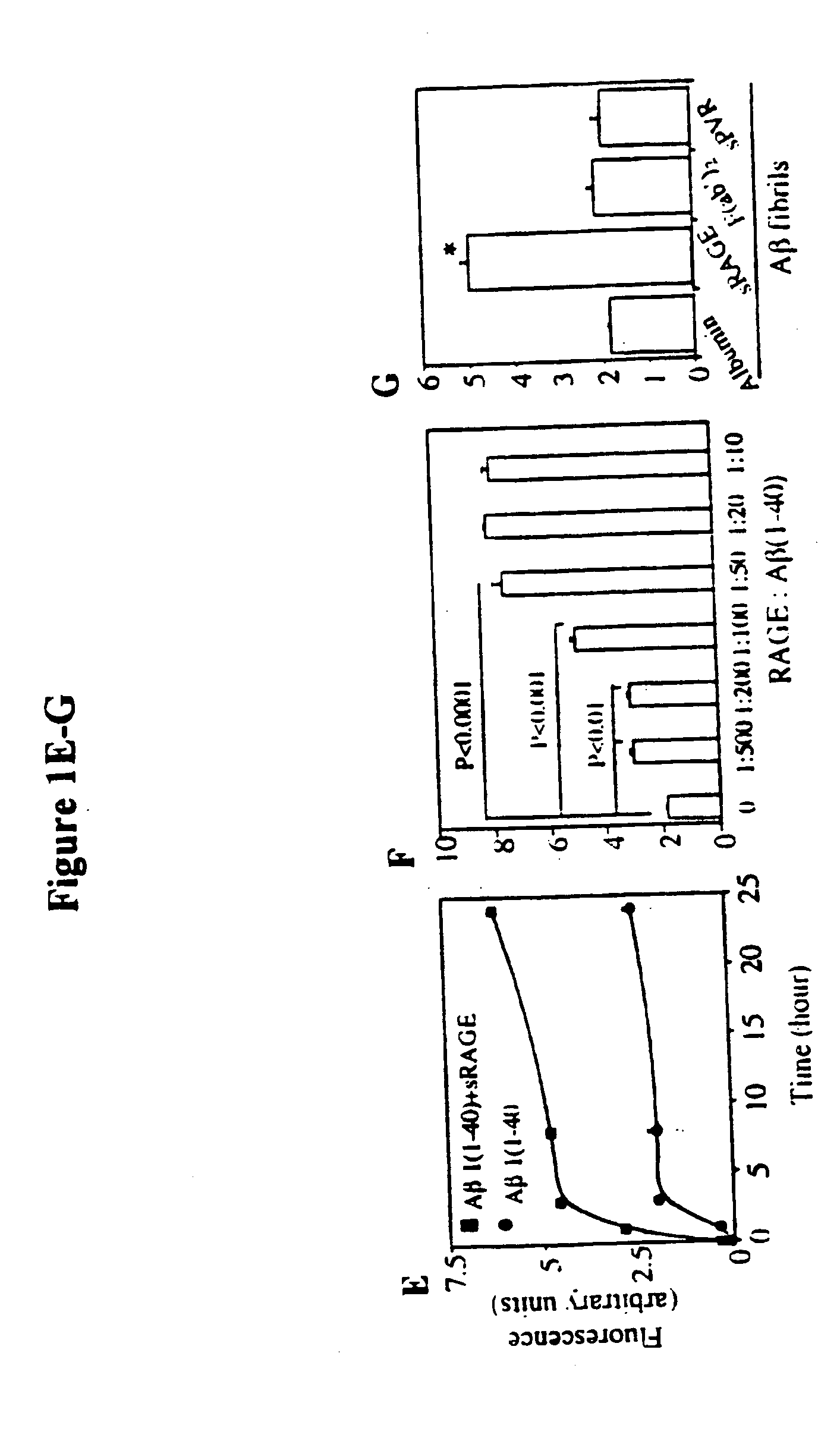
[0044]Abbreviations: Aβ, amyloid β-peptide; AD, Alzheimer's disease; AEF / SN, amyloid enhancing factor / silver nitrate; AGE, advanced glycation endproducts; βAPP, β-amyloid precursor protein; EMSA, electrophoretic mobility shift assay; HO-1, heme oxygenase type 1; IL, interleukin; ERK, Extracellular signal-regulated protein kinase; GST, glutathione-S-transferase; MAP kinase, mitogen-activated protein kinase; M-CSF, monocyte-colony stimulating factor; MEK, mitogen-activated protein kinase; NF-kB, nuclear factor kB; SAA, serum amyloid A; sRAGE, soluble RAGE; RAGE, receptor for AGE; TD, tail-deletion; wt, wild-type.
[0045]This invention provides a method of inhibiting the binding of a β-sheet fibril to RAGE on the surface of a cell which comprises contacting the cell with a binding inhibiting amount of a compound capable of inhibiting binding of the β-sheet fibril to RAGE so as to thereby inhibit binding of the β-sheet fibril to RAGE.
[0046]In one embodiment, the β-sheet fibril is amyloid ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentrations | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com