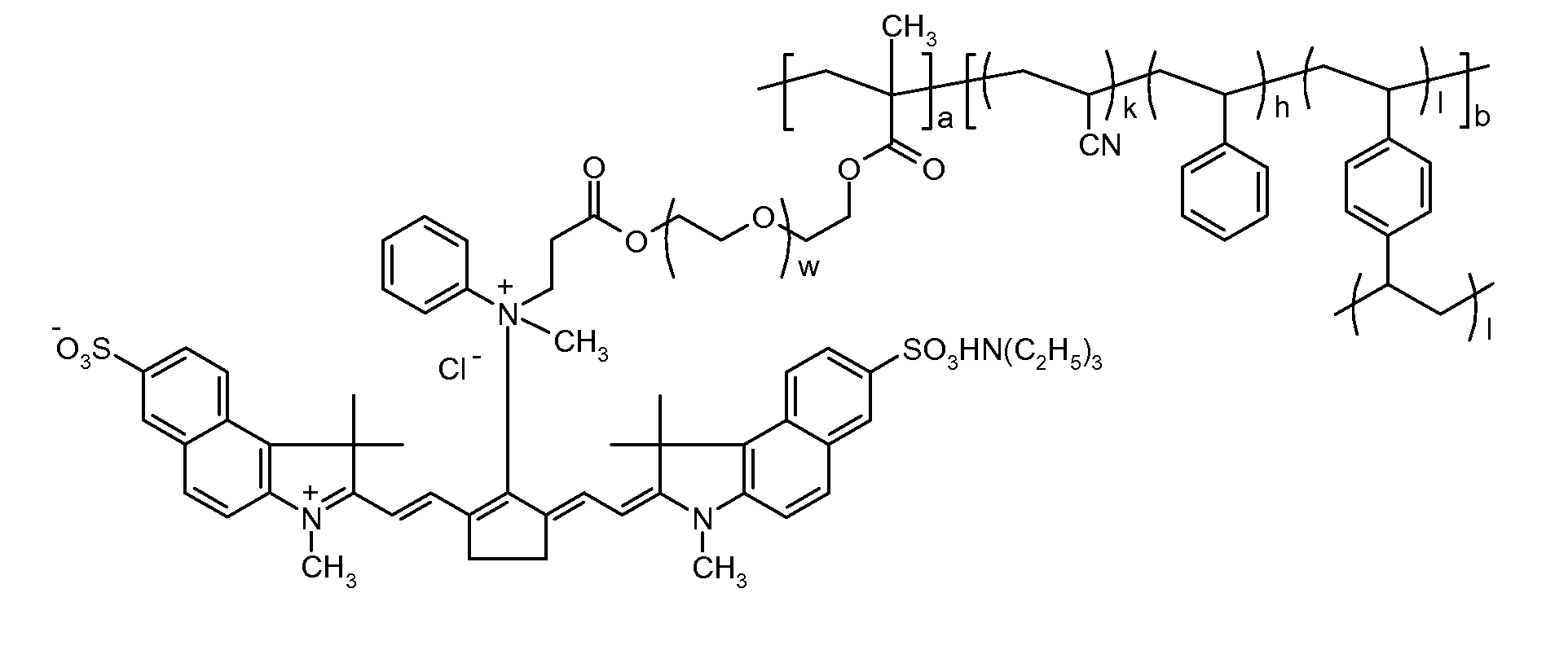
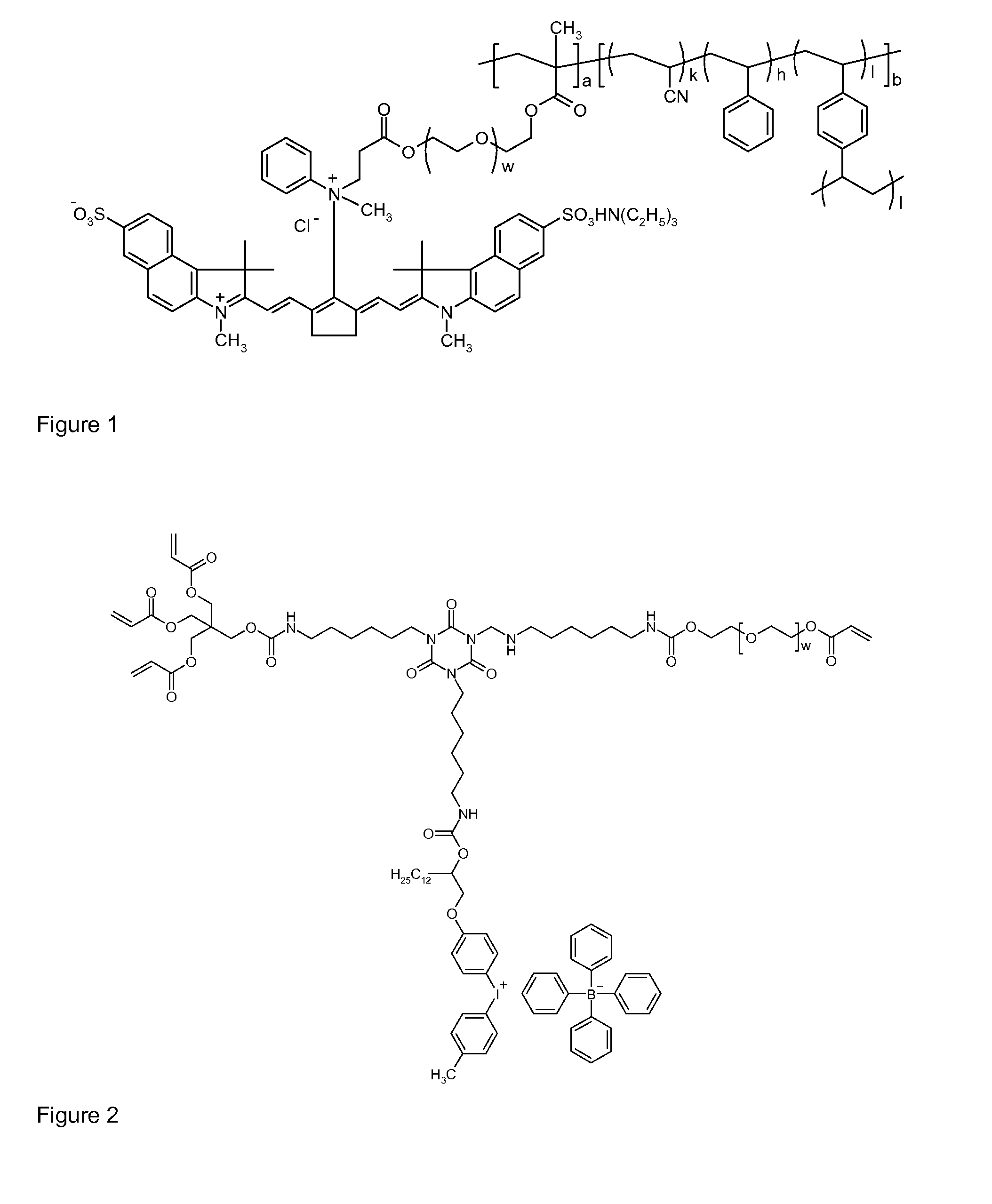
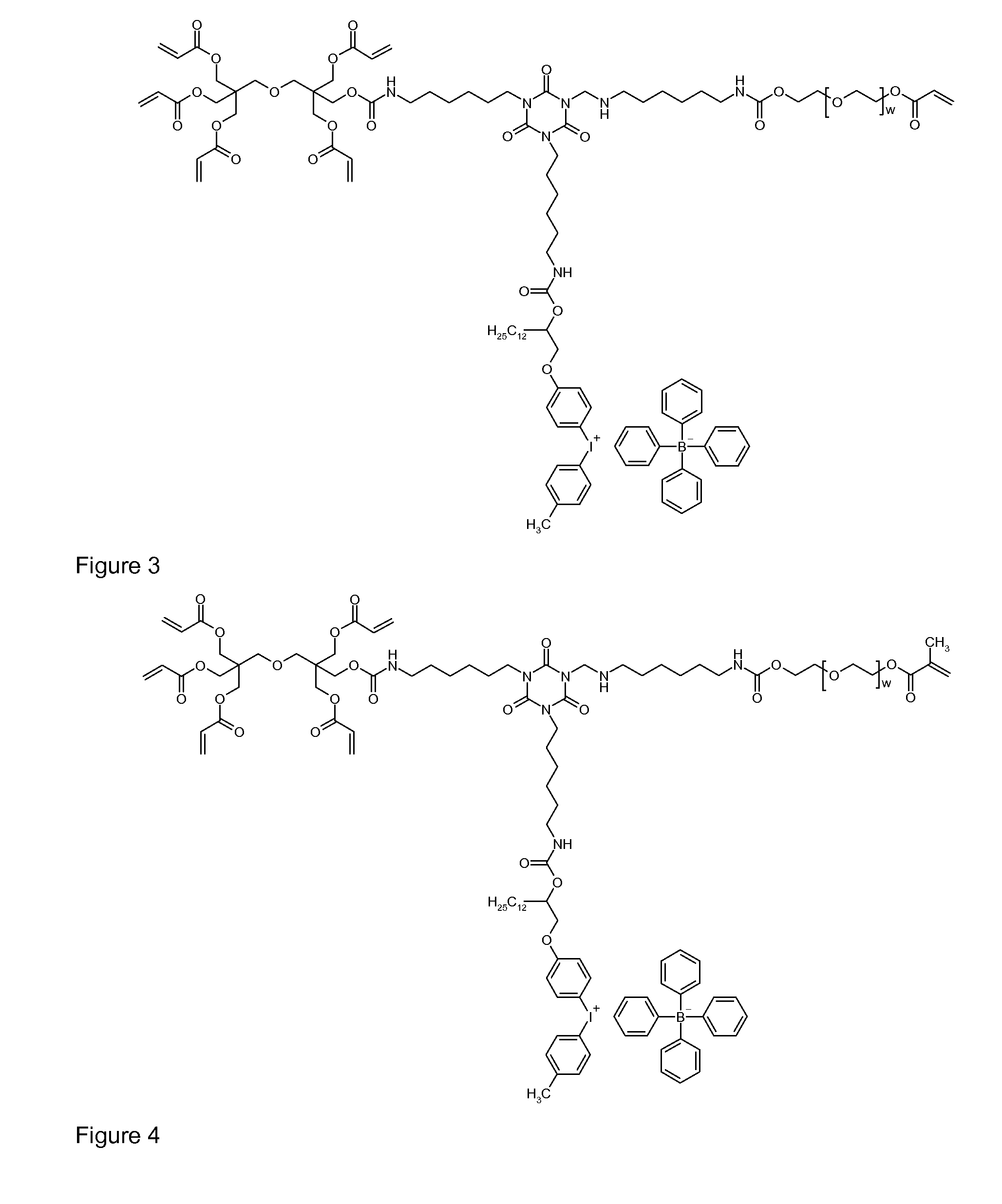
Polymeric dyes, overcoat compositions and thermal lithographic printing plates
a technology of polymeric dyes and compositions, applied in printing, printing, ball sports, etc., can solve the problems of increased costs, inability to handle or process plates under natural light, and severe background staining
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0116]Water-soluble polymeric dye, PD1-01 was synthesized by adding 84.7 grams (0.9 moles) of 1-vinylimidazole and 3.68 grams (0.1 moles) of Disperse Red 1 Acrylate into 700 ml of de-ionized water in a three neck flask. The solution was heated at 80° C. under nitrogen atmosphere for 30 minutes. Under constant stirring, 10 grams of 2,2′-azobis(2-methylpropionamidine) dihydrochloride (which acts as a free radical initiator) suspension in water were added and the solution was refluxed for 1 hour. Subsequently, 5 grams of 2,2′-azobis(2-methylpropionamidine) dihydrochloride were added and the solution was again refluxed for one hour. Finally, 5 grams of 2,2′-azobis(2-methylpropionamidine) dihydrochloride were added and the solution was heated at 80° C. for 10 hours.
[0117]A reddish solution of polymeric dye was obtained and the solid content was adjusted to 10% with de-ionized water. The maximum absorption band of the obtained polymeric dye was observed at around 503 nm. The obtained prod...
example 2
[0118]Water-soluble polymeric dye, PD1-02 was synthesized by adding 100.0 grams (0.9 moles) of 1-vinyl-2-pyrrolidone and 4.0 grams (0.1 moles) of Disperse Red 13 Acrylate into 700 ml of de-ionized water in a three neck flask. The solution was heated at 80° C. under nitrogen atmosphere for 30 minutes. Under constant stirring, 10 grams of 2,2′-azobis(2-methylpropionamidine) dihydrochloride suspension in water were added and the solution was refluxed for 1 hour. Subsequently, 5 grams of 2,2′-azobis(2-methylpropionamidine) dihydrochloride were added and the solution was again refluxed for one hour. Finally, 5 grams of 2,2′-azobis(2-methylpropionamidine) dihydrochloride were added and the solution was heated at 80° C. for 10 hours.
[0119]A reddish solution of polymeric dye was obtained and the solid content was adjusted to 10% with de-ionized water. The maximum absorption band of the obtained polymeric dye was observed at around 503 nm. The obtained product was ready for use in the prepar...
example 3
[0120]Water-soluble polymeric dye, PD1-03, was synthesized by adding 64.8 grams (0.95 moles) of acrylic acid and 4.2 grams (0.05 moles) of Direct Red 81 Methacrylate into 700 ml of de-ionized water in a three neck flask. The solution was heated at 80° C. under nitrogen atmosphere for 30 minutes. Under constant stirring, 10 grams of 2,2′-azobis(2-methylpropionamidine) dihydrochloride suspension in water were added and the solution was refluxed for 1 hour. Subsequently, 5 grams of 2,2′-azobis(2-methylpropionamidine) dihydrochloride were added and the solution was again refluxed for one hour. Finally, 5 grams of 2,2′-azobis(2-methylpropionamidine) dihydrochloride were added and the solution was heated at 80° C. for 10 hours.
[0121]A reddish solution of polymeric dye was obtained and the solid content was adjusted to 10% with de-ionized water. The maximum absorption band of the obtained polymeric dye was observed at around 503 nm. The obtained product was ready for use in the preparation...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Nanoscale particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com