Oral Controlled Release Formulation for Sedatives and Hypnotic Agents
a technology of sedatives and hypnotic agents, which is applied in the direction of drug compositions, biocide, heterocyclic compound active ingredients, etc., can solve the problems of reducing the effect of bilayer tablets such as ambien cr by at least 20%, and not maintaining the effect long enough for patients to obtain the recommended eight hours of sleep, etc., to achieve the effect of reducing auc, cmax and/or, and improving tmax
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
N=48 (Non-Fasting)
[0048]
Ln-Transformed DataLeast Squares90% Confidence LevelMeanGeometric Mean(Lower Limit, UpperPK VariableTestReferenceTestReference% RatioLimit)Cmax5.0034.855148.80128.33115.95(108.66, 123.74) AUC0-t6.4636.462641.19640.27100.14(93.56, 107.19)AUC0-∞6.4776.475650.09649.04100.16(93.64, 107.14)Non-Transformed DataLeast Squares Mean90% Confidence LevelPK VariableTestReference% Ratio(Lower Limit, Upper Limit)Cmax156.84136.64114.78(108.23, 121.34) AUC0-t705.03704.84100.03(92.83, 107.23)AUC0-∞715.28716.0399.90(92.71, 107.08)Tmax2.913.5581.85(72.22, 91.48) ke0.28450.2806101.37(96.08, 106.66)t1 / 22.822.9196.68(90.07, 103.29)
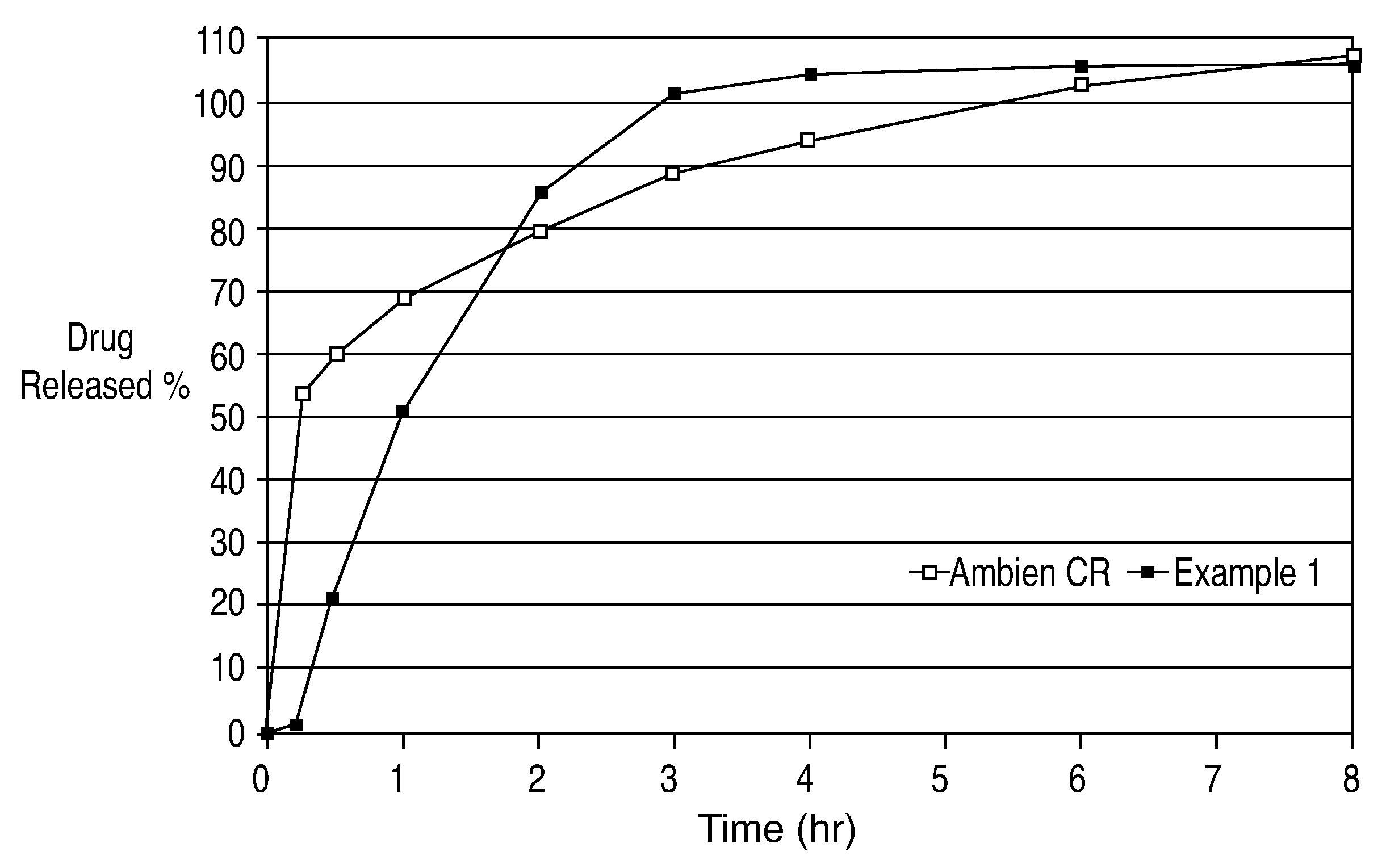
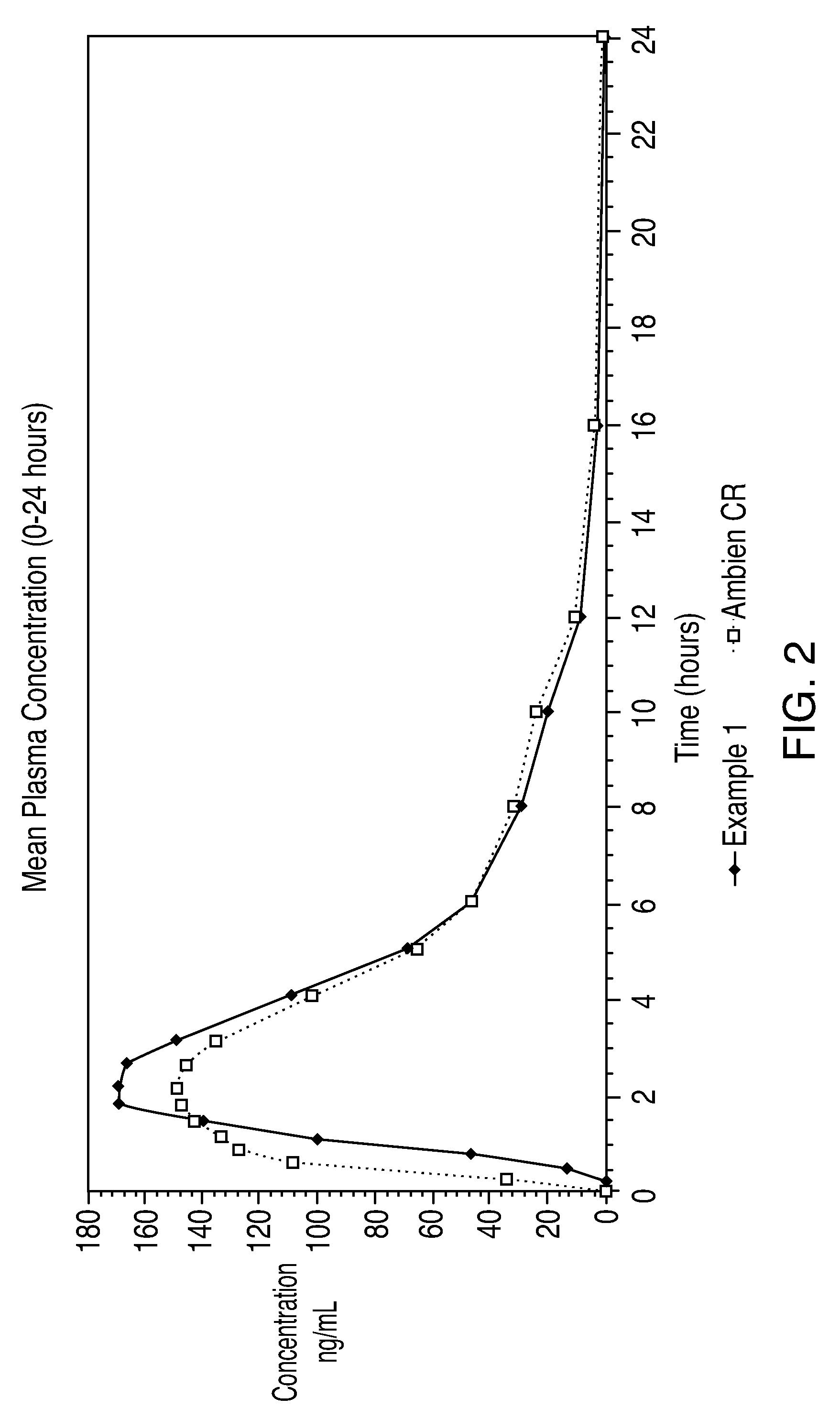
[0049]Graphs of the mean plasma concentrations based upon the above biostudies are shown in FIGS. 2 and 3.
EXAMPLE 2
[0050]A 12.5 mg zolpidem tartrate tablet in accordance with the present invention was prepared as follows:
(a) Core
[0051]3.6 kg of microcrystalline cellulose (AVICEL PH 102), 1.44 kg of hydroxypropyl methylcellulose (METHOCEL K4M Premium CR ...
example 2
N=12 (Non-Fasting)
[0057]
Ln-Transformed DataLeast Squares90% Confidence LevelMeanGeometric Mean(Lower Limit, UpperPK VariableTestReferenceTestReference% RatioLimit)Cmax4.8534.776128.14118.58108.06(85.75, 136.19)AUC0-t6.2626.400524.38602.1387.09(71.37, 106.27)AUC0-∞6.2776.429532.31619.5785.91(69.55, 106.13)Non-Transformed DataLeast Squares Mean90% Confidence LevelPK VariableTestReference% Ratio(Lower Limit, Upper Limit)Cmax139.27125.59110.89(88.01, 133.78)AUC0-t582.42656.4788.72(69.77, 107.67)AUC0-∞590.59681.3686.68(65.37, 107.98)Tmax3.423.4698.80(74.07, 123.52)ke0.32640.3033107.61(91.61, 123.62 t1 / 22.362.6190.42(68.98, 111.85
[0058]Graphs of the mean plasma concentrations based upon the above biostudies are shown in FIGS. 4 and 5.
EXAMPLE 3
[0059]A 12.5 mg zolpidem tartrate tablet in accordance with the present invention was prepared as follows:
(a) Core
[0060]9.266 kg of microcrystalline cellulose (AVICEL PH 102), 1.875 kg of hydroxypropyl methylcellulose (METHOCEL K4M Premium CR Grad...
example 3
N=11 (Fasting)
[0066]
Ln-Transformed DataIntervalLeast SquaresMean(LowerPKMeanGeometric MeanSquareLimit, UpperVariableA: TestReferenceA: TestReference% RatioErrorLimit)Cmax5.2715.137194.62170.25114.320.02967(100.65, 129.85)AUC0-t6.6566.708777.40819.0194.920.3196 (83.17, 108.33)AUC0-∞6.6676.720785.89829.0994.790.03114(83.19, 108) Non-Transformed DataIntervalPKLeast Squares Mean(Lower Limit, UpperVariableA: TestReference% RatioMean Square ErrorLimit)Cmax206.28183.62112.341143.88(98.72, 125.96)AUC0-t822.43898.2991.5621199.75(79.57, 103.34)AUC0-∞831.16908.3991.5021151.39(79.66, 103.34)Tmax1.841.46125.650.9654(75.92, 175.38)ke0.26210.2317113.130.00106(102.75, 123.50) t1 / 22.773.2286.180.1475(77.36, 95.01)
[0067]A graph of the mean plasma concentration based upon the above biostudy is shown in FIG. 6.
EXAMPLE 4
[0068]A 12.5 mg zolpidem tartrate tablet in accordance with the present invention was prepared as follows:
(a) Core
[0069]9.375 kg of microcrystalline cellulose (AVICEL PH 102), 1.563 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| weight percent | aaaaa | aaaaa |
| weight percent | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com