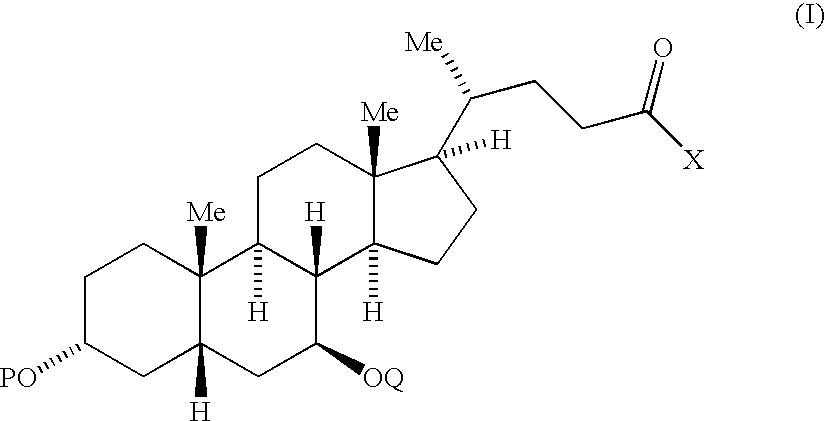
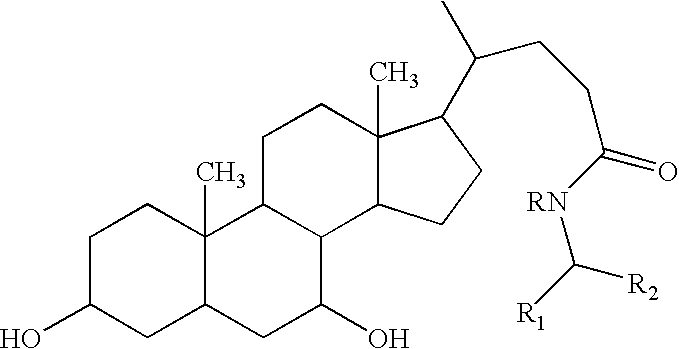
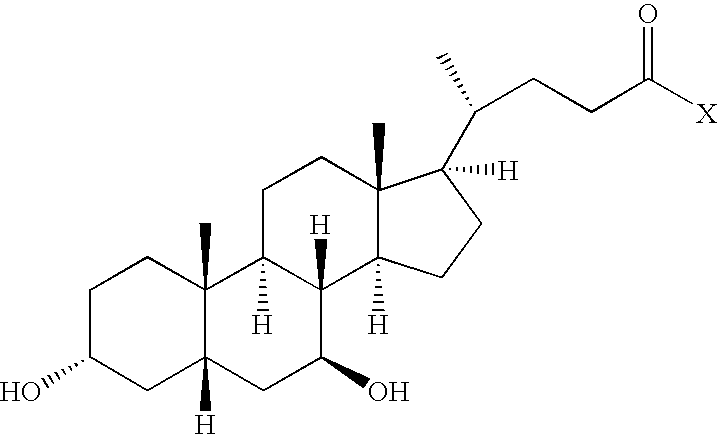
Compounds and methods for treatment of disorders associated with er stress
a technology of er stress and compounds, applied in the field of compounds and methods for treating disorders associated with er stress, can solve the problems of hypercholesterolemia, a growing health problem, and cholesterol-associated diseases becoming serious threats to human health, and achieve the effect of preventing complications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
ERSE / URSE Activity Assay
[0369]The following assay may be used to identify compounds that will be useful in treating conditions related to ER stress.
[0370]When ER stress is triggered, a number of signaling events called unfolded protein response (UPR) is activated. One of the downstream effects of UPR is to increase transcriptional activity of genes with the promoter regions containing the endoplasmic reticulum stress element (ERSE) and unfolded response element (UPRE). One of UPR's eventual goals is to make more of those chaperone proteins needed for protein folding and destruction and thereby to increase functional capacity.
[0371]Accordingly, the compounds of the present invention may be analysed using this phenomena. For example, the results of screening test compounds may be expressed as percent induction of the ERSE reporter gene relative to tunicamycin treatment. Moreover, analysis may be made on ratios of reporter activity (firefly luciferase) / pCMV-RL (renilla luciferase) with...
example 2
[0391]
Soft Gelatin Capsule Formulation IItemIngredientsmg / Capsule1.Compound10.001-0.022.Butylated Hydroxytoluene (BHT)0.0163.Butylated Hydroxyanisole (BHA)0.0164.Miglyol 812 qs.160.0
Manufacturing Procedure:
[0392]1. BHT and BHA is suspended in Miglyol 812 and warmed to about 50° C. with stirring, until dissolved.
[0393]2. A compound of the invention is dissolved in the solution from step 1 at 50° C.
[0394]3. The solution from Step 2 is cooled at room temperature.
[0395]4. The solution from Step 3 is filled into soft gelatin capsules.
Note: All manufacturing steps are performed under a nitrogen atmosphere and protected from light.
example 3
[0396]
Soft Gelatin Capsule Formulation IIItemIngredientsmg / Capsule1.Compound of the invention10.001-0.022.di-α-Tocopherol0.0163.Miglyol 812 qs.160.0
Manufacturing Procedure:
[0397]1. Di-α-Tocopherol is suspended in Miglyol 812 and warmed to about 50° C. with stirring, until dissolved.
[0398]2. A compound of the invention.
[0399]3. The solution from Step 2 is cooled at room temperature.
[0400]4. The solution from Step 3 is filled into soft gelatin capsules.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com