Methods and collagen products for tissue repair
a collagen product and tissue repair technology, applied in the direction of peptide/protein ingredients, ligaments, prostheses, etc., can solve the problems of premature loss of fibrin clot scaffold, meniscus and articular cartilage in human joints that fail to heal after an injury, and disrupt the healing process of tissues within the joint or intra-articular tissues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Collagen solution and testing of Properties
[0160]A. Minimal Gelation Achieved with Some Formulations
[0161]1. Innocoll: Aliquots of Innocoll Collagen (Starting pH=4.1) were Made.
[0162]Weights of aliquots were 0.380 mg collagen, pH=4.1. One half of the samples had 5 microliters NaHCO3 added to bring pH to between 7.0 and 8.0 and one half were not neutralized. 300 microliters fetal bovine serum was added to each aliquot. All solutions were monitored for gelation at 37° C. for 30 minutes. The solutions remained liquid, with viscosities similar to that of water (approximately 1 centipoise). No increase in viscosity with time was noted over the hour long period.
[0163]Identical experiments with Innocoll aliquots at starting pH=2.5 were also performed. Additionally, experiments were performed using ratios of collagen:FBS of 1:1, 2:1 and 3:1. None of these materials produced a gelled product at 37° C. The solutions remained liquid, with viscosities similar to that of water (ap...
example 2
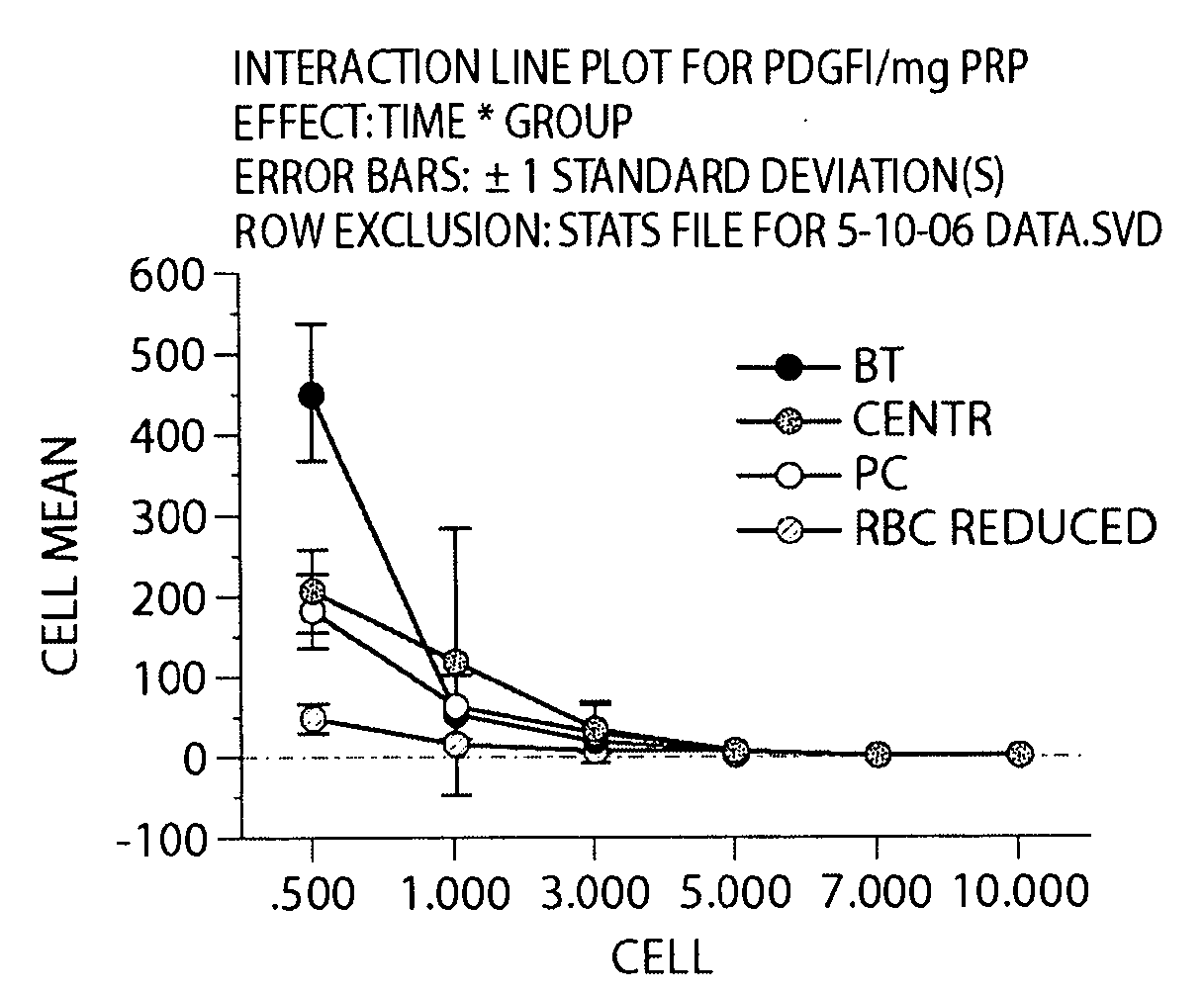
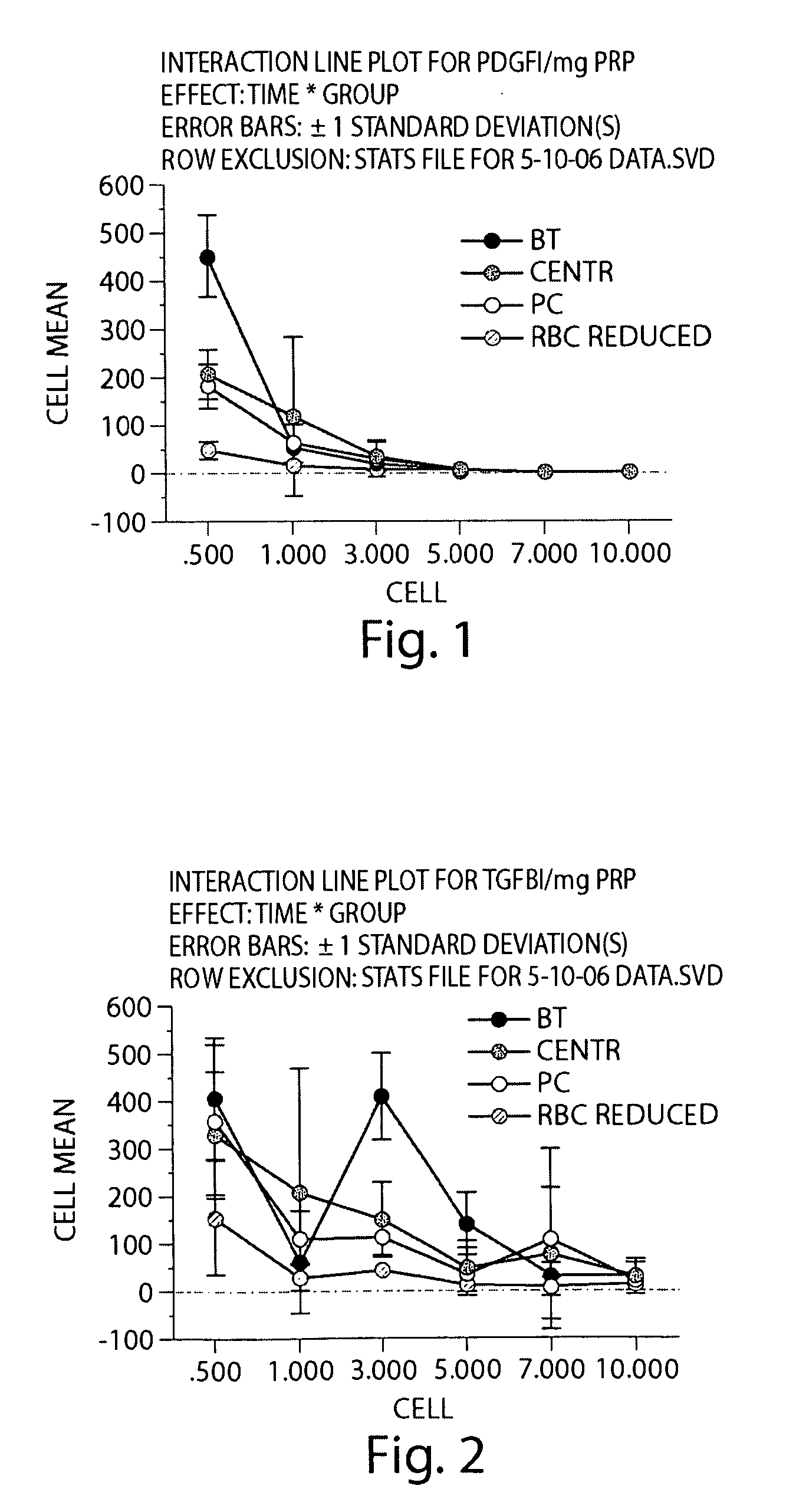
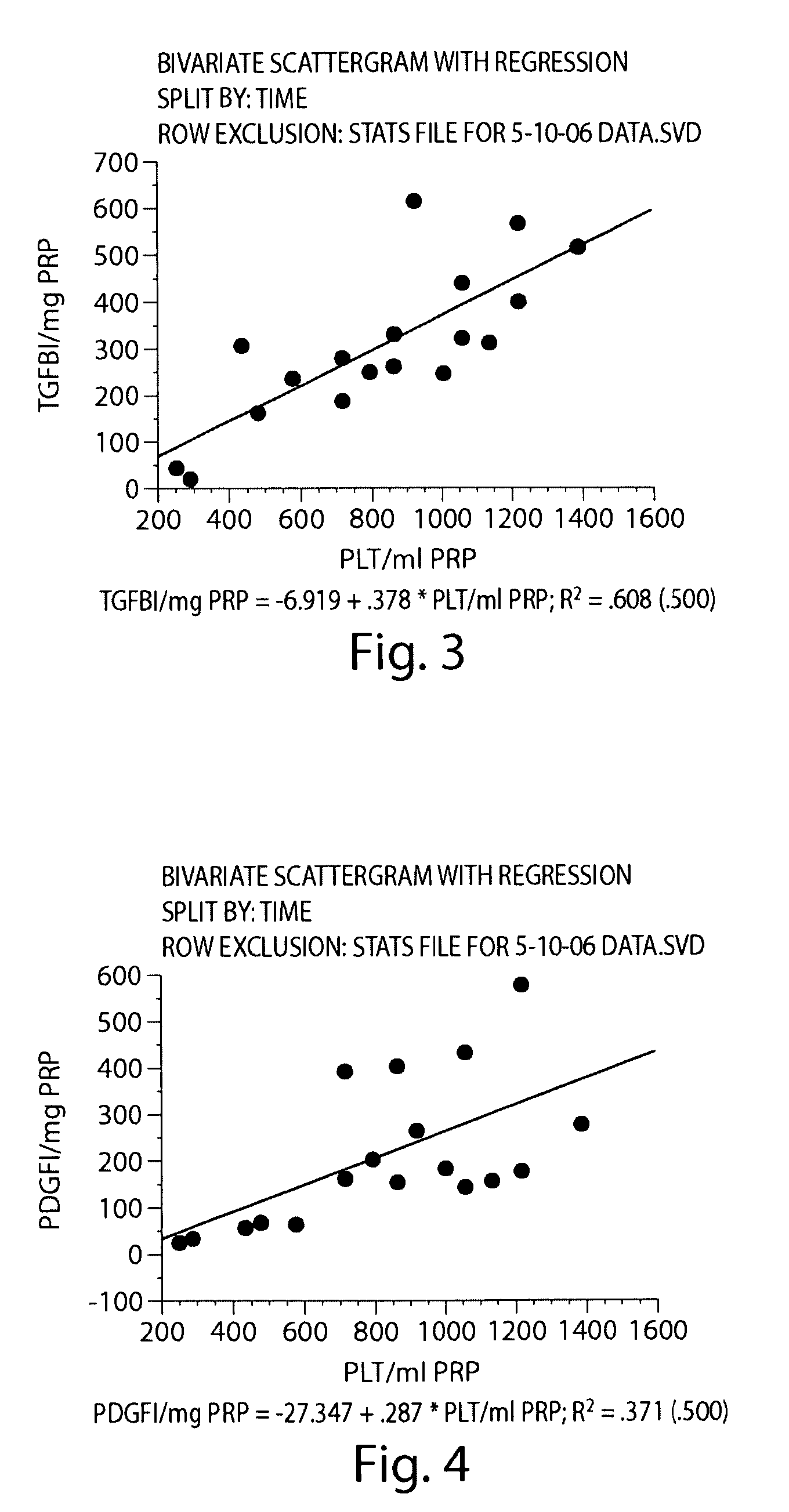
[0194]In this Example, type I collagen was used to stimulate activation of the fibrin clotting mechanism and platelet activation. Initially, we tested collagen to determine if it would result in more sustained release of two growth factors used as markers of platelet function, namely TGF-β1 and PDGF-αβ, when compared with the use of exogenous thrombin. Secondly, we tested whether the amount of this release would be dependent on the platelet concentration in the PRP. The release profiles of these growth factors from three types of collagen-PRP gels were compared with the release profile from a PRP clot created with exogenous bovine thrombin over 10 days. Additionally, we determined whether the growth factor release from collagen-activated PRP hydrogels would cause a physiologic changes in ACL cells in terms of 1) cellular metabolism of growth factors, 2) cellular proliferation within the gels and 3) cell-mediated gel contraction.
Materials and Methods
Preparation of Platelet-Rich Plasm...
example 3
[0231]The components of a preferred collagen solution of the invention was tested to identify components.
Methods
[0232]Collagen from various sources (Cellagen, MP Biomedicals, Solon, Ohio (shown as lane 4 in FIG. 11); Elastin Products Company, Inc., Owensville, Mo. (shown as lane 5 in FIG. 11); StemCell Technologies (shown as lane 6 in FIG. 11), Becton Dickinson, Franklin Lakes, N.J. (shown as lane 7 in FIG. 11); fluorescein-labeled collagen (shown as lane 8 in FIG. 11)) were prepared into aliquot samples. Total protein content of each collagen sample was determined using a calorimetric assay (BCA Protein Assay Kit, Rockford, Ill.) in order to aliquot samples containing equal protein content. The aliquots were treated with SDS and β-mercaptoethanol and placed at 100° C. for five minutes for denaturation and then loaded onto a 4-12% SDS-PAGE gel. The gels were stained with Coomassie Blue (Bio-Rad, Hercules, Calif.) and washed with a 7.5% acetic acid and 5% methanol destaining solution...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentrations | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com