Spent fuel reprocessing method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
first embodiment
[0020]The first embodiment of spent fuel reprocessing method according to the present invention will be described below by referring to FIGS. 1 and 2.
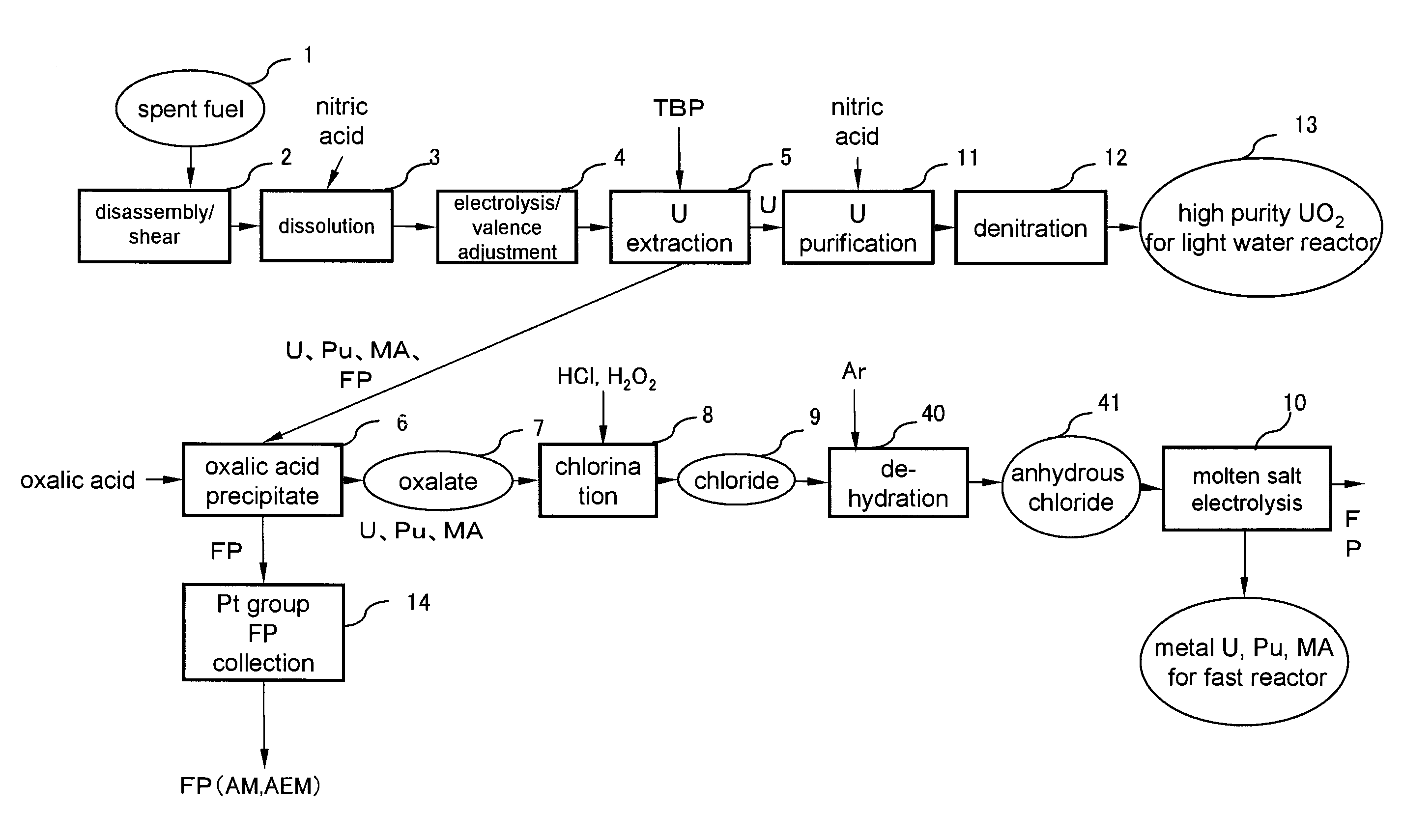
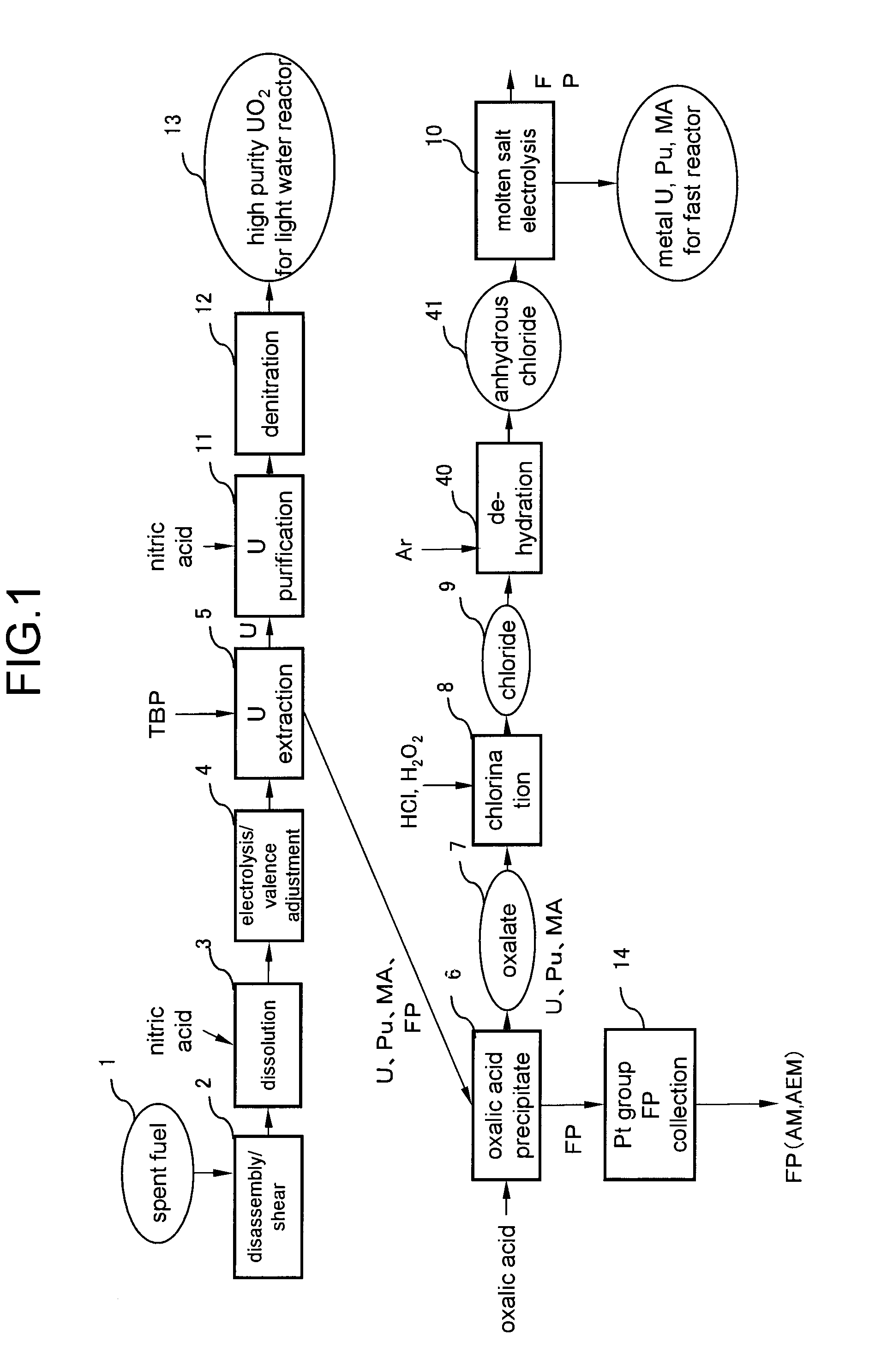
[0021]FIG. 1 is a flowchart of spent fuel reprocessing method according to a first embodiment of the present invention. Referring to FIG. 1, first, spent oxide fuel 1 is disassembled and sheared in a disassembly / shear step 2. Subsequently, all the spent oxide fuel is dissolved by nitric acid in a dissolution step 3. At this time, U exists in a hexavalent state whereas Pu exists in a tetravalent state.
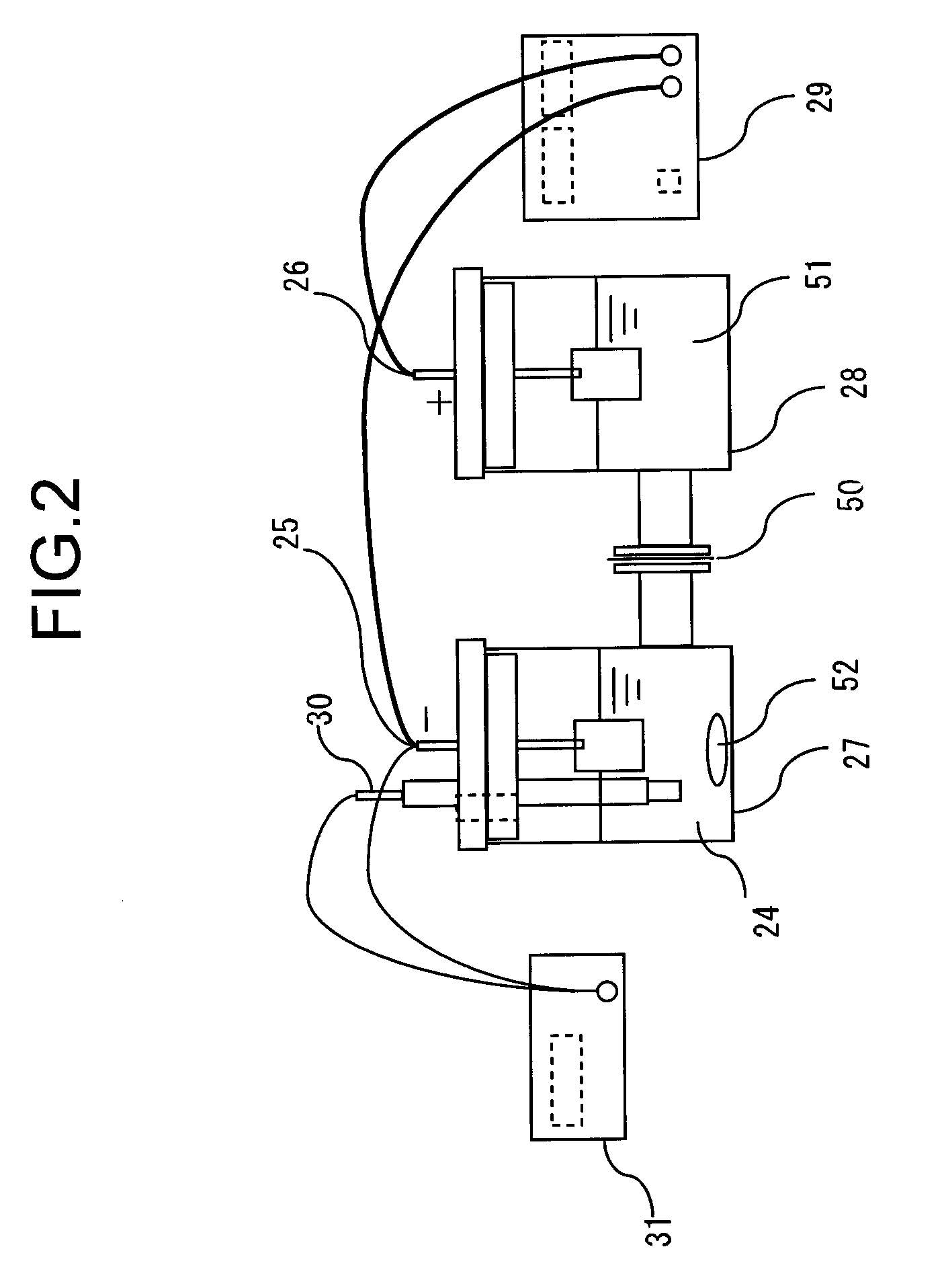
[0022]Thereafter, Pu is electrolytically reduced to trivalent in an electrolysis / valence adjustment step 4. FIG. 2 is a schematic sectional elevational view of an apparatus that can be employed for the electrolysis / valence adjustment step 4 of the first embodiment. More specifically, a cathode chamber 27 and an anode chamber 28 are separated from each other by means of a diaphragm 50 in the apparatus. Catholyte 24 is stored in the cathode ...
second embodiment
[0038]Now, the second embodiment of spent fuel reprocessing method according to the present invention will be described below by referring to FIGS. 5 and 6. The parts of this embodiment that same as or similar to those of the first embodiment are denoted respectively by the same reference symbols and will not be described repeatedly.
[0039]FIG. 5 is a flowchart of spent fuel reprocessing method according to a second embodiment of the present invention. FIG. 6 is a schematic sectional elevational view of an apparatus that can be employed for the electrolysis / reduction step of the second embodiment.
[0040]The sequence down to the oxalic acid precipitation step 6, where the oxalic acid precipitate 7 containing U, Pu, minor actinides and rare earth elements are collected, is same as that of the first embodiment.
[0041]This second embodiment has an oxidation / dehydration step 15 and an electrolysis / reduction step 17 instead of the chlorination step 8, the dehydration step 40 and the molten s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Electric potential / voltage | aaaaa | aaaaa |
| Current density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com