Methods and Materials for the Detection of Leishmania Infection
a technology of leishmania and detection methods, applied in the field of leishmania diagnosis, can solve the problems of lack of sensitivity and specificity of tests, and the current diagnostic procedures are not readily applicable to field situations, and achieve the effect of rapid treatmen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Monoclonal Antibodies to Leishmania Amastigotes
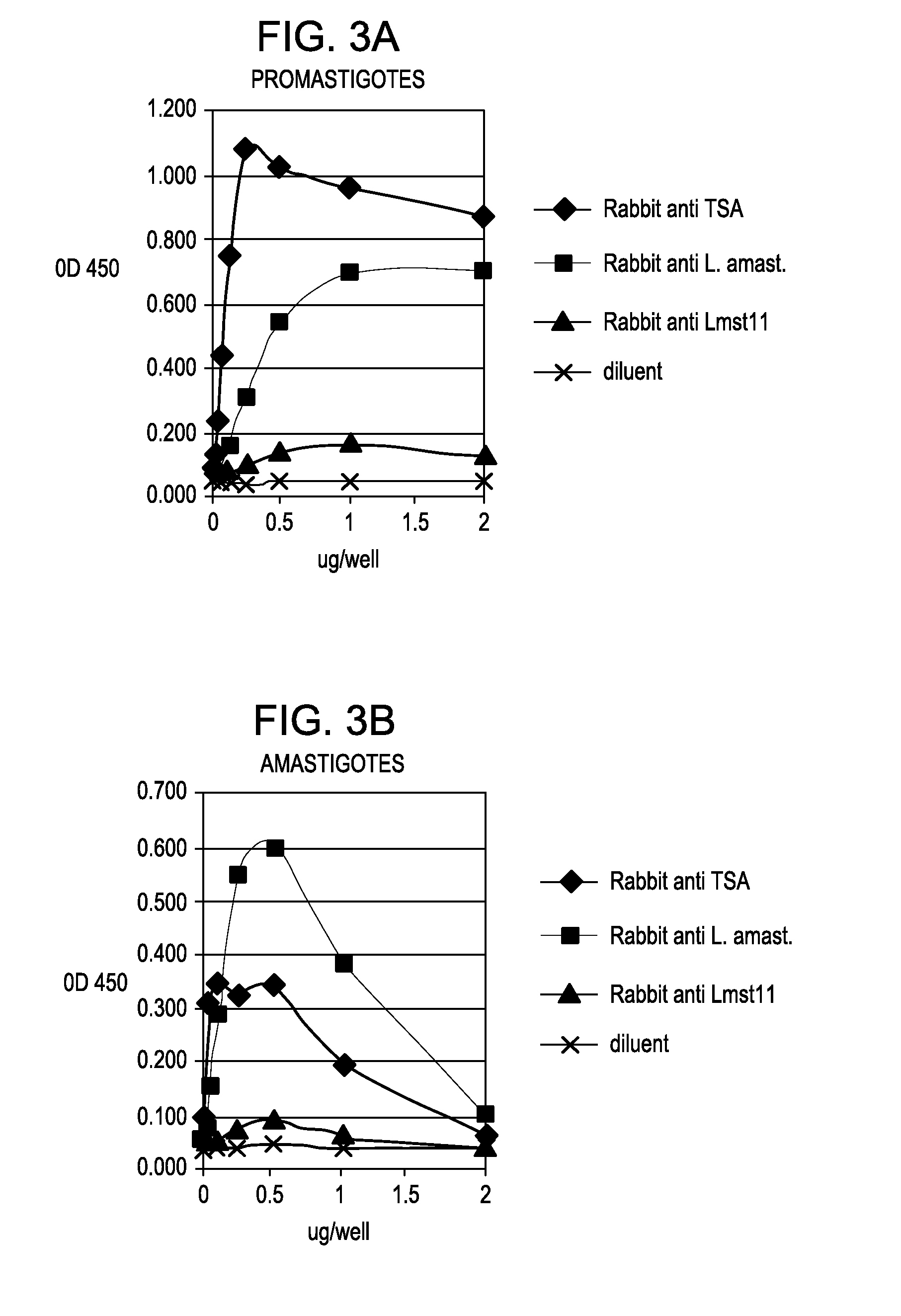
[0048]Amastigotes for immunization were isolated from non-necrotic lesions at the dorsal part of the tail base in L. major RM2-infected RAG2− / − mice approximately 4 to 5 weeks after infection. The sonicated amastigotes were centrifuged at 1,600 g for 30 min at 4° C. and the resulting supernatants were used as antigens. BALB / c mice were immunized with whole lysate of amastigotes with complete Freund's adjuvant. After the first immunization, the mice were inoculated with the same dose of amastigote homogenate every 3 weeks. The mice were given a final boost of amastigote homogenate 4 days before fusion. Hybrid cells were produced by fusing P3-X63-Ag8-U1 cells with spleen cells isolated from the immunized mice. Hybridomas were screened for production of antibodies against amastigote antigens by ELISA. Limiting dilution was performed twice to obtain monoclonal hybridomas. One of the hybridomas, referred to as C11C, was inocul...
example 2
Preparation of Monoclonal and Polyclonal Antibodies to Recombinant TSA
[0049]Recombinant TSA expressed in E. coli was used as immunogen for preparing additional monoclonal antibodies in mice and for raising a polyclonal antibody in rabbits. Supernatants from four monoclonal antibodies were tested for their reactivity against TSA and an unrelated T. cruzi protein as negative control. In addition, the rabbit polyclonal antibody was affinity purified on a TSA sepharose column for use in ELISA and rapid test development.
example 3
Reactivity of Antibodies Against L. Major Amastigotes and Promastigotes
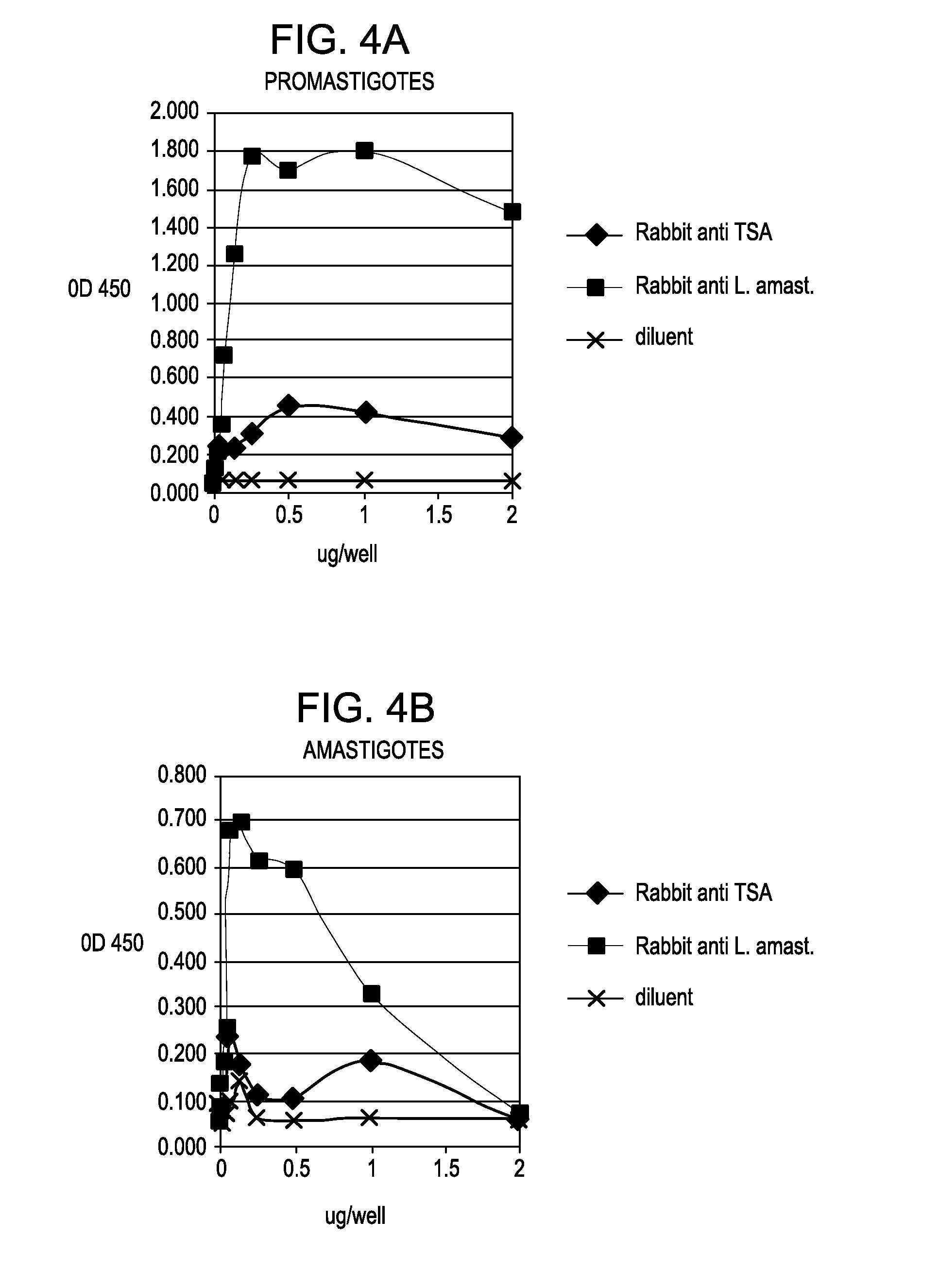
[0050]Various antibodies, including C11C, were analyzed to determine their reactivity with solubilized preparations of L. major promastigotes grown in culture, as well as with amastigotes derived from skin lesions of mice infected with L. major. These studies enabled the selection of antibodies / target antigens that reacted with both forms of L. major but with particular emphasis on amastigotes, the form present in skin lesions. We initially analyzed antibodies to test their reactivity / titer with a fixed concentration of antigen on an ELISA plate and then, using a fixed dilution, determined what level of antigen could be detected in both L. major promastigotes and amastigotes, the latter being derived from skin lesions from infected mice. Polyclonal antibodies used in these initial studies were against L. major amastigotes, TSA, LmSTI1 (M15), HSP83 (JV91), HSP70 (JV8E), LACK antigen and a secreted polyprotein from...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com