Method and composition for treatment of skeletal dysplasias
a technology for skeletal dysplasia and composition, applied in the field of compositions for the treatment of skeletal dysplasia, can solve the problems of skeletal dysplasia (sd), and achieve the effect of enhancing np stabilization in circulation and increasing stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
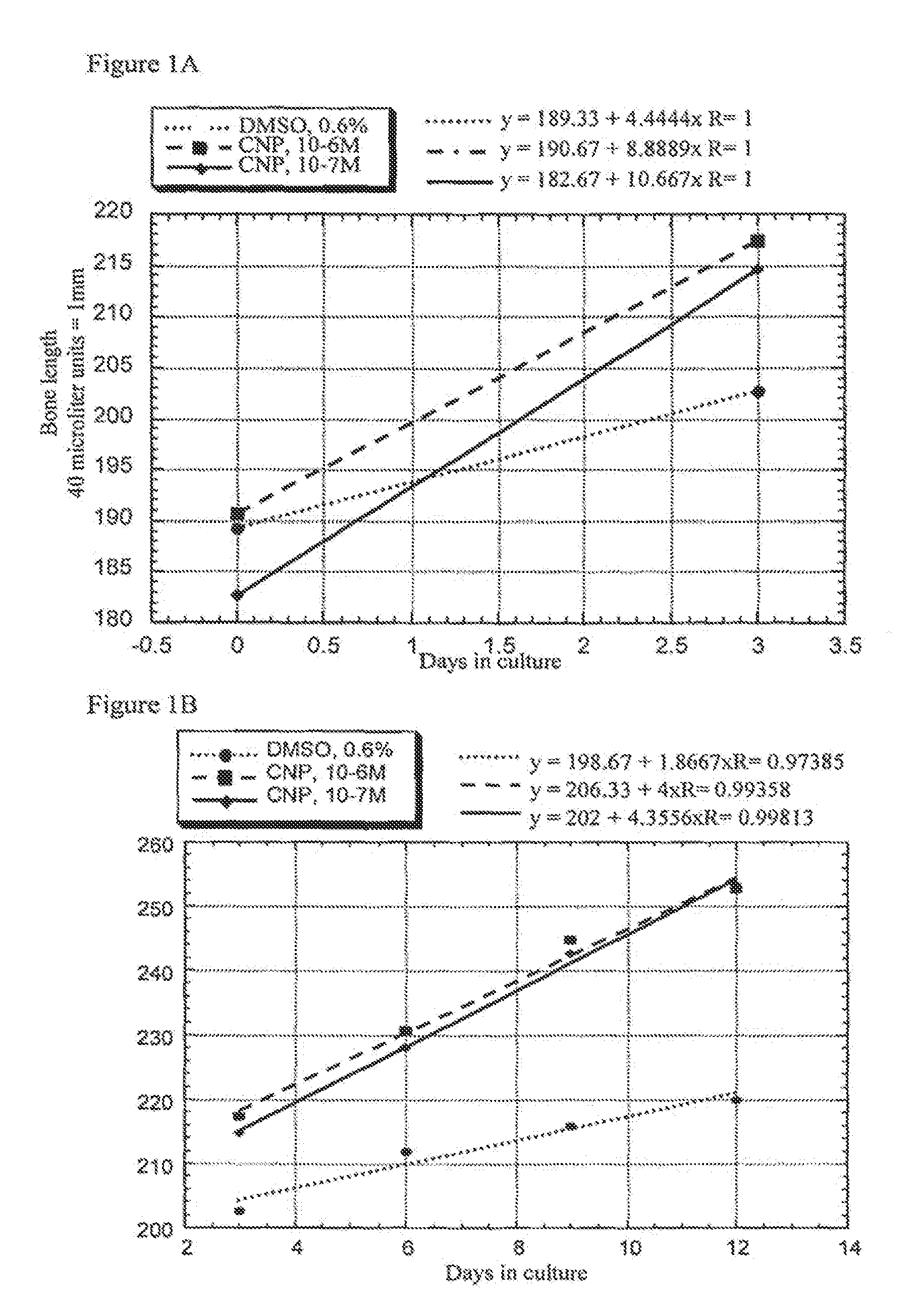
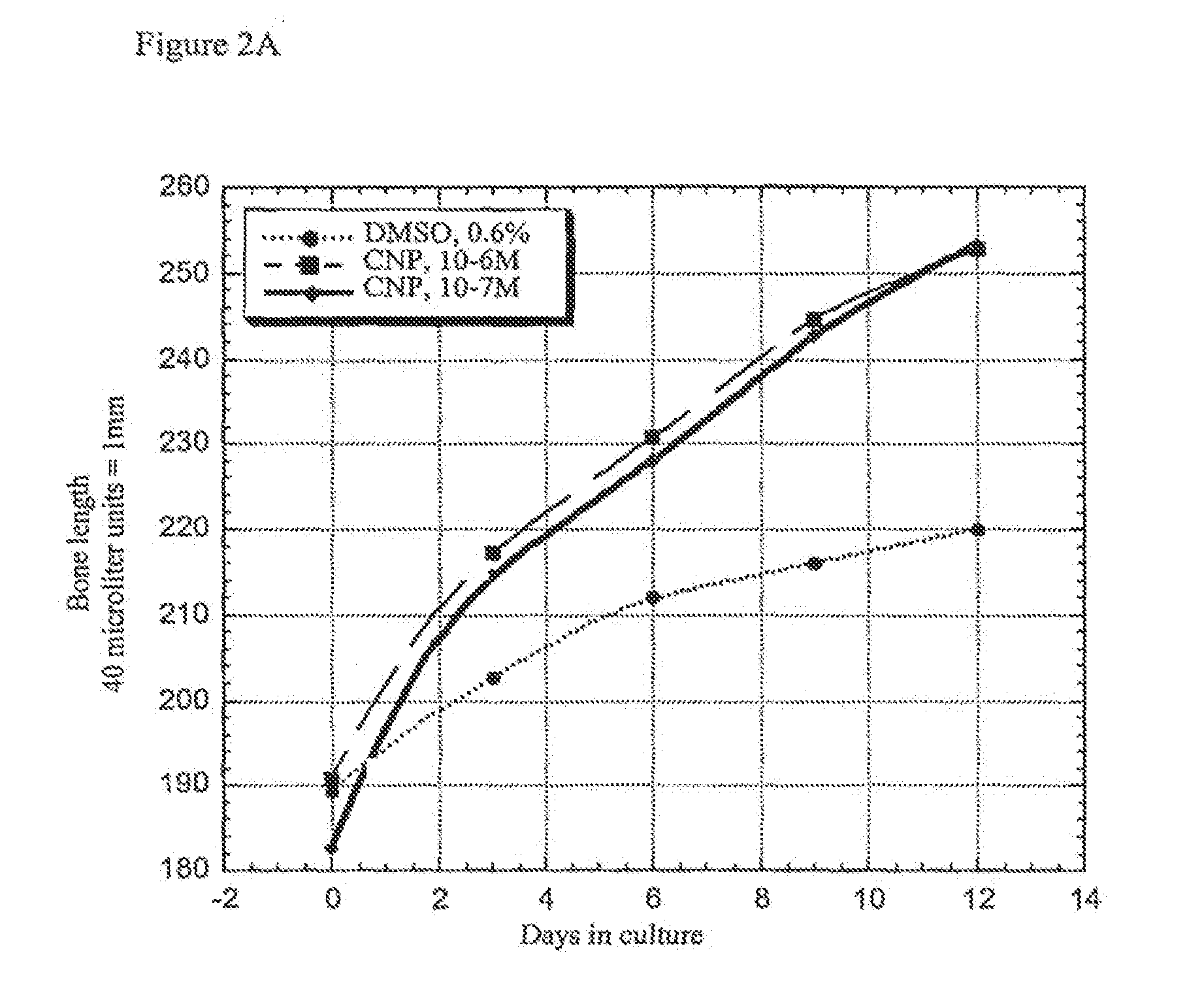
Ex Vivo Bone Culture
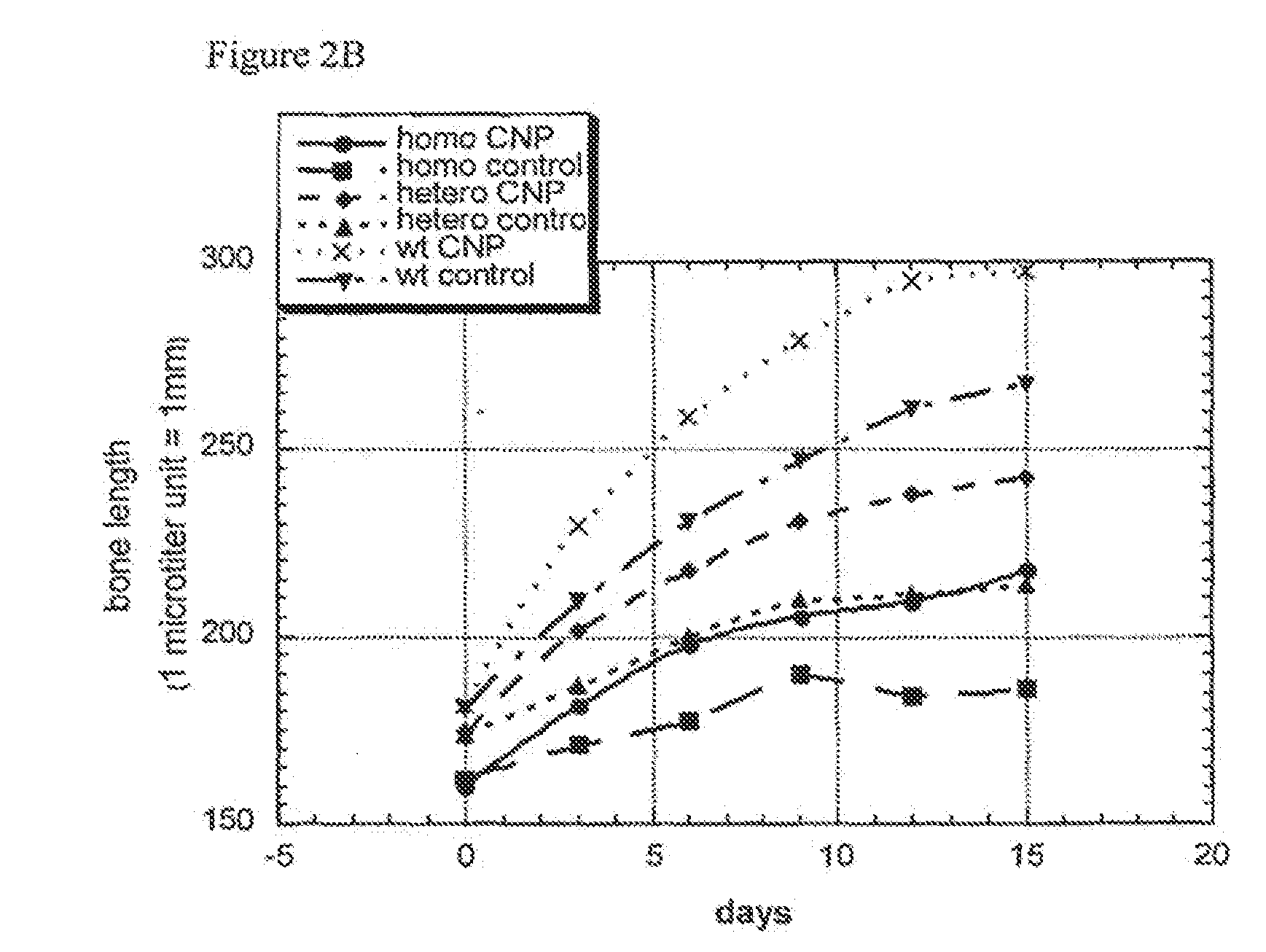
[0079]Femora derived from achondroplasia model mice (Ach369 knock-in mice carry the Gly to Cys mutation at position 369, analogous to position 375 in humans or Ach374 knock-in mice that carry the Gly to Arg mutation at position 374, analogous to position 380 in humans) were dissected from P0 wild type, heterozygote and homozygote littermates and cultured in the presence of natriuretic peptides for 15 days.
[0080]Protocol for bone culture: Femoral bone cultures were performed by excising the hind limbs of mice. The limbs were carefully cleaned from the surrounding tissue (skin and muscles) and the femora exposed. The femora were removed and further cleared from tissue remains and ligaments. The femora were measured for their initial length, using a binocular with an eyepiece micrometer ruler. The bones were grown in 1 ml of medium (α-MEM supplemented with penicillin (100 units / ml), streptomycin (0.1 mg / ml), nystatin (12.5 units / ml), BSA (0.2%), O-glycerophosphate (...
example 2
Natriuretic Peptides Lead to an Increase in the Size of Growth Plate
Morphological and Immunohistological Analysis
[0085]Most of the elongation induced by natriuretic peptides observed in bone culture results from epiphyseal growth and not from elongation of the mineralized diaphysis. Morphological analysis of the cellular composition of the growth plate in treated samples revealed that there is an increase in the size of the proliferative region and in the size of the hypertrophic zone. In addition, there is an area of a cellular matrix similar to that observed in the growth plate of FGFR3 knockout mice. Therefore, there seems to be an increase in proliferation which leads to a larger growth plate.
[0086]Mouse-specific BNP was synthesized and labeled with biotin. Biotinylated BNP was tested in bone culture on bones derived from Achondroplasia mice and shown to induce bone elongation. Nevertheless, a higher concentration of BNP was needed. Histological analysis of the distribution of b...
experiment 3
In vivo Administration
1. IP Administration of Drugs to Achondroplasia Pups
[0088]The fastest growth period for mice is during the first month of life therefore, it is expected that the earlier the experiments are initiated, the greater the influence on growth. The disadvantage of treating such young animals is that there is a limit on volume delivered. Drug delivery cannot be performed intravenously (IV) or by implantation of a continuous delivery device like a pump.
[0089]Animals: Heterozygote mice for the achondroplasia mutation, aged P4-P5, weighing approximately 3.5 gr. were randomly separated into groups of 3 animals / group and ear marked. Animals were distributed 6-7 individuals / cage with one foster mother.
[0090]Materials and Procedure: Animals were injected daily with drug intraperitoneally (IP) in a volume not exceeding 50 μl. This volume can be increased proportionally to weight increase. Animals are weighed and the tail length is measured on day 0 and every 2 days thereafter....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com