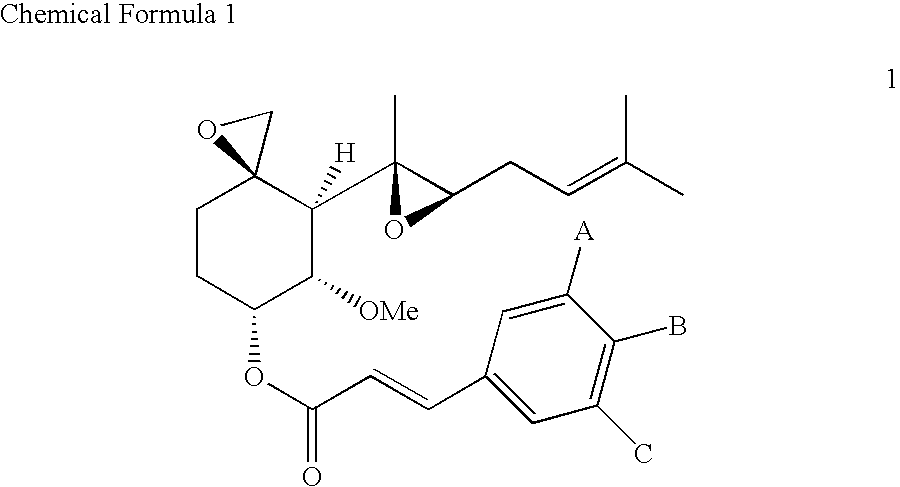
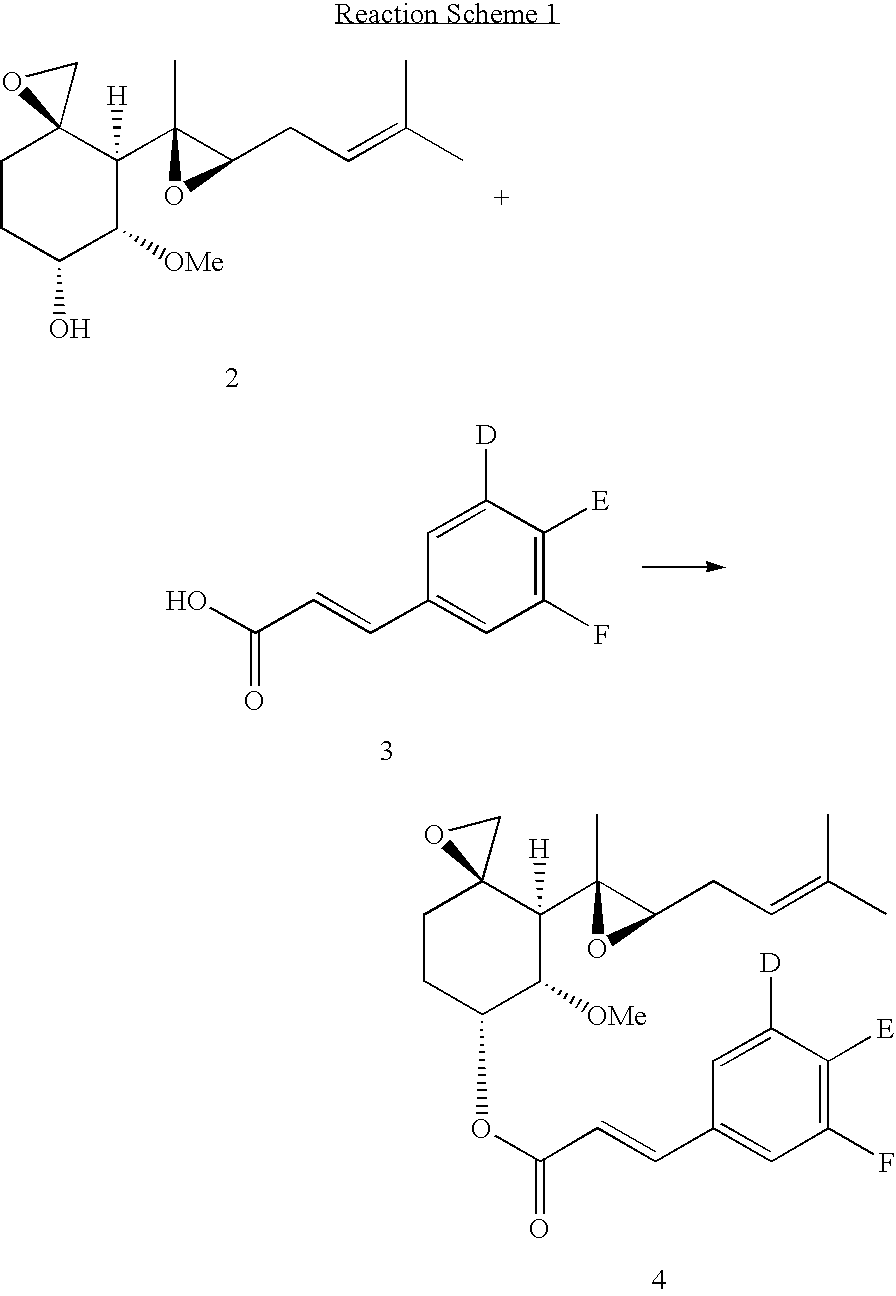
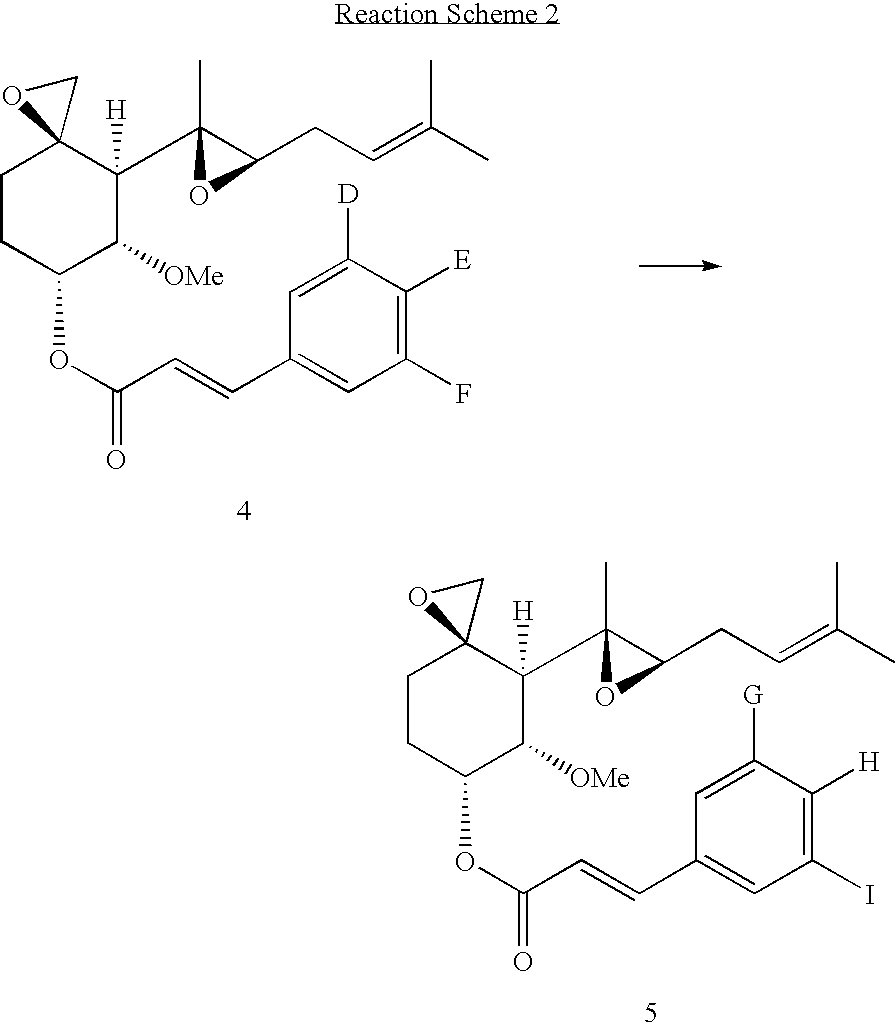
Fumagillol derivatives or method for preparation of fumagillol derivatives, and pharmaceutical compositions comprising the same
a technology of fumagillol and derivatives, which is applied in the field of fumagillol derivatives, can solve the problems of chemical stability, toxicity, and few possibilities of tolerance occurring, and achieve the effects of improving toxicity and chemical stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
A preparation of O-(3,5-dimethoxy-4-(2-hydroxyethoxy)cinnamoyl)fumagillol
Step 1: A preparation of O-(4-acetoxy-3,5-dimethoxycinnamoyl)fumagillol
[0091]The same procedure as described in the Step 1 of the Example 1 was repeated, but using a compound of the Chemical Formula 2 (1.0 g), 4-acetoxy-3,5-dimethoxycinnamic acid (2.36 g), thionylchloride (1.29 ml), toluene (20 ml), sodium hydride (850 mg) and dimethylformamide (20 ml), to give 1.36 g (72%) of the title compound as a white solid.
[0092]1H-NMR (400 MHz, CDCl3) δ: 7.59 (d, 1H, J=16 Hz), 6.77 (s, 2H), 6.44 (d, 1H, J=16 Hz), 5.71 (m, 1H), 5.21 (m, 1H), 3.86 (s, 3H), 3.71 (dd, 1H, J=11, 2.7 Hz), 3.45 (s, 3H), 3.0 (d, 1H, J=4.0 Hz), 2.62 (t, 1H, J=6.4 Hz), 2.57 (d, 1H, J=4.0 Hz), 2.36 (m, 1H), 2.34 (s, 3H), 2.20-2.04 (m, 4H), 1.89 (m, 1H), 1.74 (s, 3H), 1.65 (s, 3H), 1.23 (s, 3H), 1.10 (m, 1H).
Step 2: A preparation of O-(3,5-dimethoxy-4-hydroxycinnamoyl)fumagillol
[0093]The same procedure as described in the step 2 of the Example 1 was...
example 3
A preparation of O-(4-(2-hydroxyethoxy)-3-methoxycinnamoyl)fumagillol
Step 1: A preparation of O-(4-acetoxy-3-methoxycinnamoyl)fumagillol
[0097]The sane procedure as described in the step 1 of Example 1 was repeated but using a compound of the Chemical Formula 2 (1.0 g), 4-acetoxy-3-methoxycinnamic acid (2.09 g), thionylchloride (1.29 ml), toluene (20 ml), triethylamine (2.7 ml) and dichloromethane (20 ml), to give 1.0 g (56%) of the title compound as light yellow syrub.
[0098]1H-NMR (400 MHz, CDCl3) δ: 7.62 (d, 1H, J=16 Hz), 7.13-7.03 (m, 3H), 6.44 (d, 1H, J=16 Hz), 5.73 (m, 1H), 5.43 (m, 1H), 5.21 (m, 1H), 3.88 (s, 3H), 3.71 (dd, 1H, J=11.2, 2.8 Hz), 3.45 (s, 3H), 3.00 (d, 1H, J=4 Hz), 2.62 (t, 1H, J=6.3 Hz), 2.57 (d, 1H, J=4 Hz), 2.35 (m, 1H), 2.32 (s, 3H), 2.20-2.04 (m, 4H), 1.89 (m, 1H), 1.74 (s, 3H), 1.65 (s, 3H), 1.23 (s, 3H), 1.11 (m, 1H).
Step 2: A preparation of O-(4-hydroxy-3-methoxycinnamoyl)fumagillol
[0099]The same procedure as described in the step 2 of Example 1 was repea...
example 4
A preparation of O-(3-(2-hydroxyethoxy)-4-methoxycinnamoyl)fumagillol
Step 1: A preparation of O-(3-acetoxy-4-methoxycinnamoyl)fumagillol
[0103]The same procedure as described in the step 1 of Example 1 was repeated but using a compound of the Chemical Formula 2 (1.0 g), 3-acetoxy-4-methoxycinnamic acid (2.09 g), thionylchloride (1.29 ml), toluene (20 ml), triethylamine (2.7 ml) and dichloromethane (20 ml), to give 1.01 g (59%) of the title compound as white solid.
[0104]1H-NMR (400 MHz, CDCl3) δ: 7.58 (d, 1H, J=16 Hz), 7.73 (m, 1H), 7.23 (m, 1H), 6.96 (d, 1H, J=8.5 Hz), 6.34 (d, 1H, J=16 Hz), 5.72 (m, 1H), 5.21 (m, 1H), 3.86 (s, 3H), 3.70 (dd, 1H, J=11, 2.7 Hz), 3.45 (s, 3H), 3.04 (d, 1H, J=4 Hz), 2.61 (t, 1H, J=6.3 Hz), 2.56 (d, 1H, J=4 Hz), 2.35 (m, 1H), 2.19-2.01 (m, 4H), 1.88 (m, 1H), 1.74 (s, 3H), 1.66 (s, 3H), 1.23 (s, 3H), 1.12 (m, 1H).
Step 2: A preparation of O-(3-hydroxy-4-methoxycinnamoyl)fumagillol
[0105]The same procedure as described in the step 2 of Example 1 was repeated...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com