Orally bioavailable stilbenoids- compositions and therapeutic applications thereof
a technology of stilbenoids and compositions, applied in the field of natural fat modulators, can solve the problems of totally ineffective adipogenesis of stilbenoids, and achieve the effect of inhibiting adipogenesis and enhancing the ability to prevent accumulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
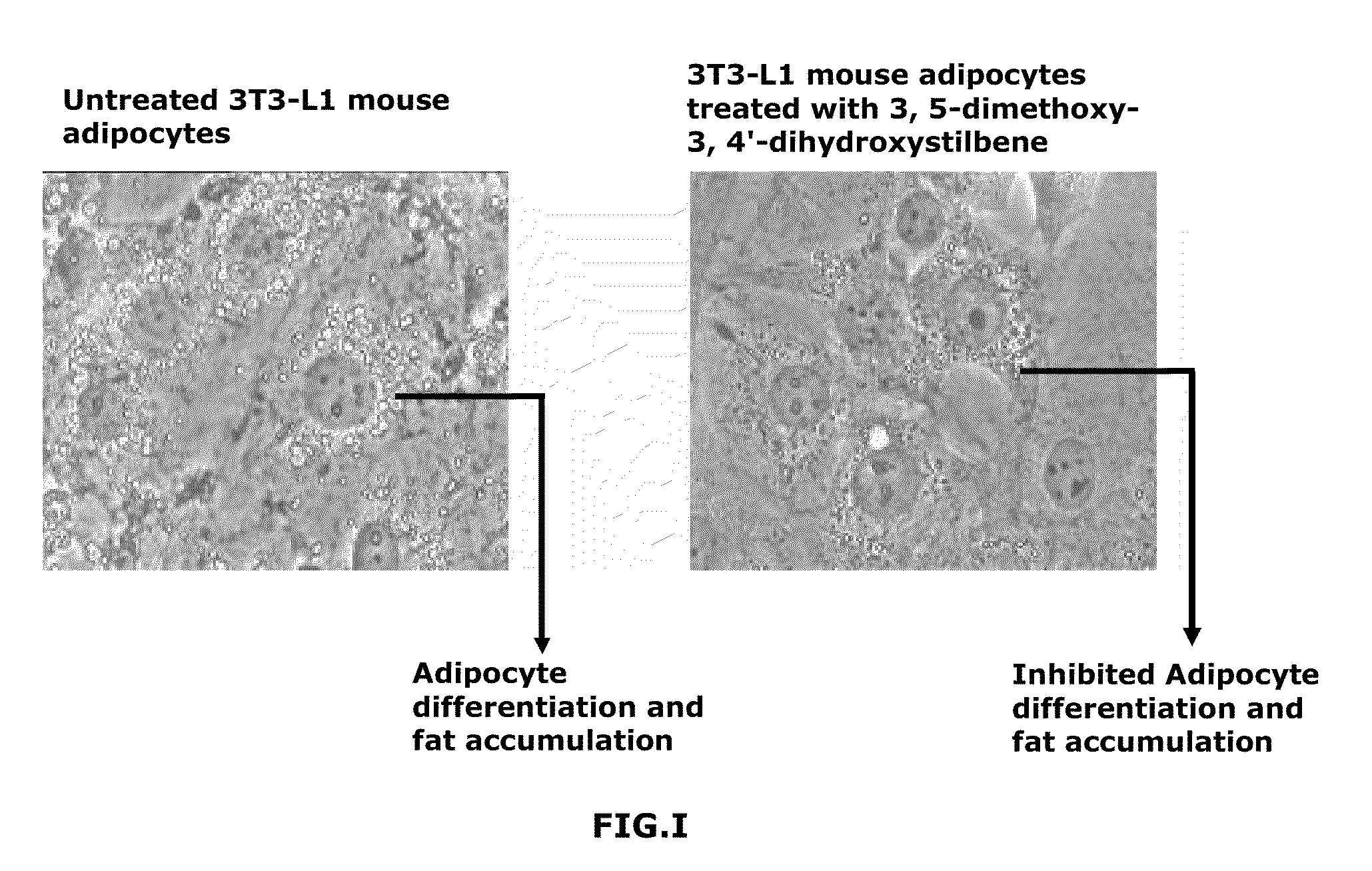
FIG. I—Adipogenesis Inhibitory Activity
[0031]Principle:
[0032]A common assay to measure adipocyte differentiation in cell culture is with the dye Oil Red-O, which is a lipid-soluble red dye. Since terminal differentiation of adipocytes is accompanied by the accumulation of great amounts of lipids in large cytoplasmic vesicles, a strong, bright, staining of the cytoplasm with this dye is a reliable indicator of adipocyte differentiation.
[0033]Methodology:
[0034]3T3-L1 mouse adipocyte cells are seeded at a density of 5000 cell / 200 μl of adipocyte induction medium in a 96 well plate. After 48 hrs varying concentrations of the sample are added. After 72 hrs, the medium is changed to adipocyte progression medium along with the sample. The medium is similarly changed after another 48 hours. The plates are washed gently after 48 hrs with 100 μl of PBS. 100 μl of 10% formalin is used to fix the cells for 30 min keeping at RT. The cells are then washed twice with 60% isopropanol gently. 100 μl...
example 2
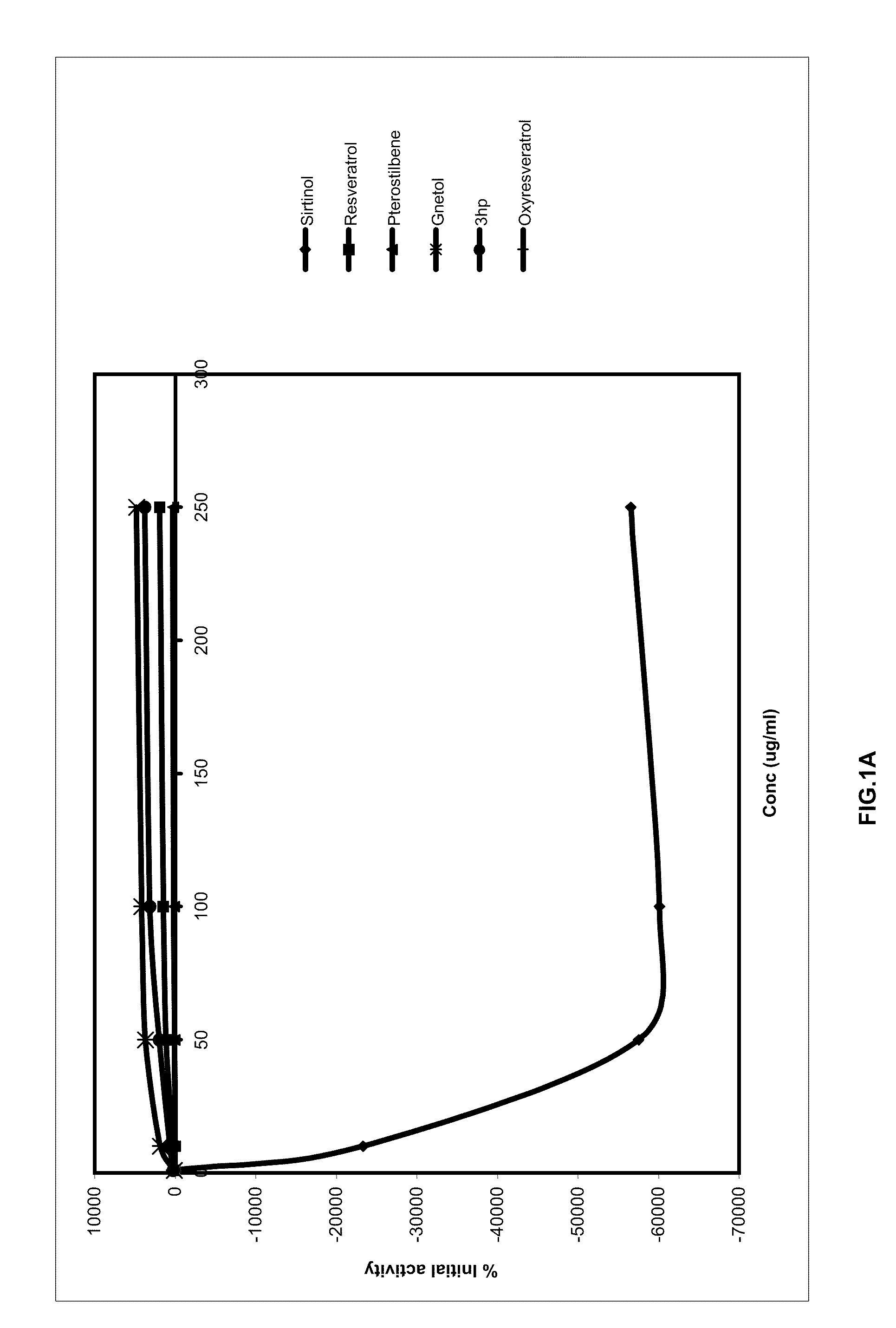
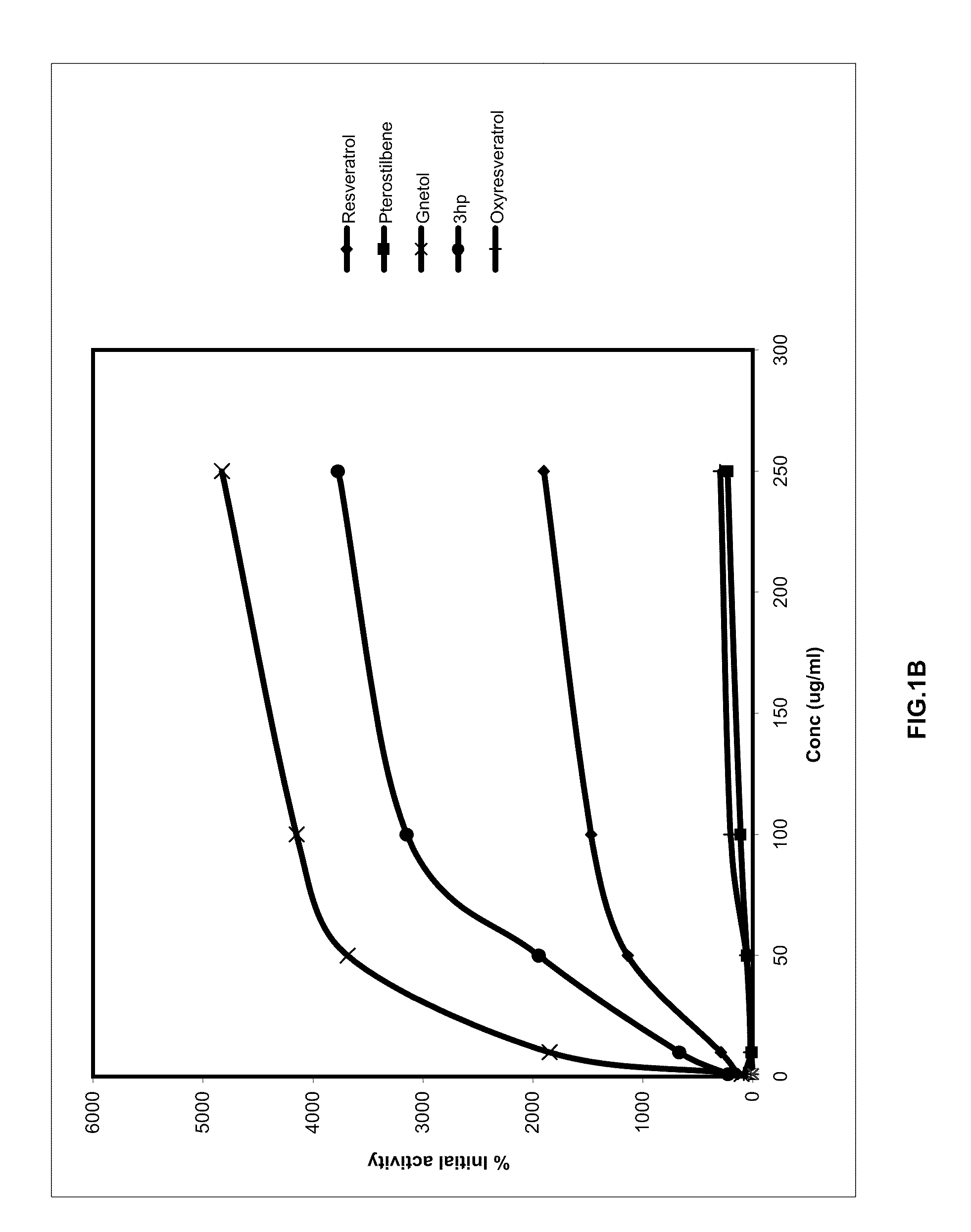
FIGS. IA and 1B
[0044]SIRT-1 activation / inhibition was measured using the SIRT 1 Direct fluorescent Screening Assay kit (Catalog No. 10010401) and instructions provided therein of Cayman Chemical Company, 1180 East Ellsworth Road, Ann Arbor, Mich. 48108 USA. (http: / / www.caymanchem.com / pdfs / 10010401.pdf)
TABLE APercentage SIRT 1 modulation by stilbenoidsSirtinol2-[(2-Hydroxynaphthalen-Sirtinol2-[(2-Hydroxynaphthalen-Concentration1-Concentrationylmethylene)amino]-3-hydroxyPterostilbene(μg / ml)N-(1-phenyl-ethyl)•benzamideResveratrolPterostilbeneGnetol(3hp)Oxyresveratrol164.44137.782.22104.44224.4477.7810−23326.67291.1111.111846.67668.8924.4450−57517.781137.7853.333686.671946.6760.00100−60093.331471.11113.334151.113146.67202.22250−56544.441902.22231.114826.673771.11297.78
[0045]Results (Table A) and Discussion
[0046]It may be known from Table A that both 3,5-dimethoxy-3,4′-dihydroxystilbene (3-hydroxypterostilbene) and 2,3′,5′,6-tetrahydroxy-trans-stilbene (gnetol) show enhanced percentage S...
example 3
Tables B, C, D and E
[0047]Antimicrobial Studies of 3-Hydroxy Pterostilbene Against Propionibacterium acnes
[0048]Objective: To compare the effect of 3-Hydroxypsterostilbene and Resveratrol on Propionibacterium acnes growth.
[0049]Propionibacterium acnes is a gram-positive, non-spore forming, anaerobic, pleomorphic rod found in clinical specimens. In human body P.acnes thrives on areas most exposed to air, such as the face and the nose. Its ability to live as an anaerobic in an air-exposed environment comes from the fact that P.acnes lives in the microhabitat sebaceous follicles thus causing acne vulgaris.
[0050]Method: Disc diffusion method was done to study Minimum inhibitory concentration (MIC).
[0051]Materials[0052]1. Propionibacterium acnes ATCC 11827[0053]2. Physiological saline or Buffered peptone water (BPW).[0054]3. Reinforced clostridium medium (RCM) Hi-media[0055]4. Reinforced clostridium agar (RCA) Hi-media[0056]5. Anaerobic chamber.[0057]6. Gassing manifold Nitrogen, mixed...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com