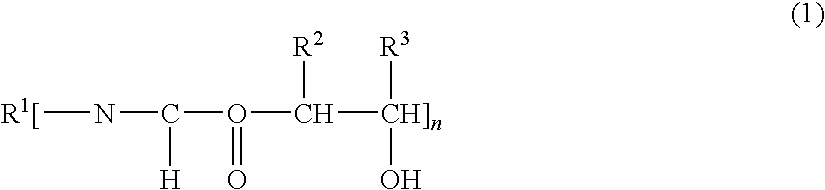
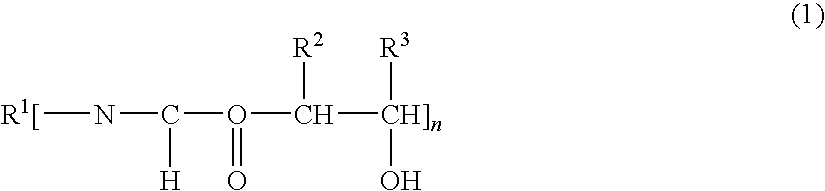
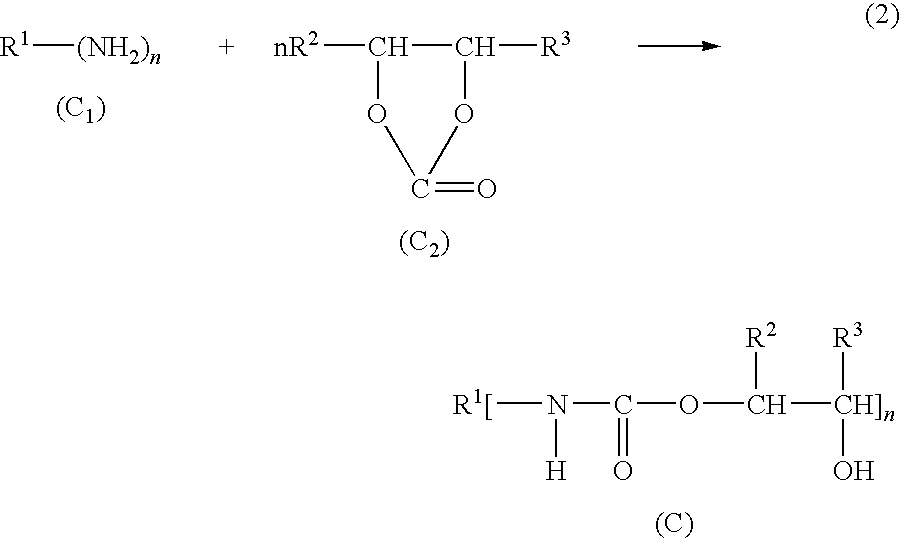
Epoxi-amine composition modified with hydroxyalkyl urethane
a technology of hydroxyalkyl urethane and epoxi-amine, which is applied in the field of epoxi-amine compositions, can solve the problems of limited influence of benzyl alcohol and acids as accelerators, inability to stabilize reactive adducts, and inability to cure epoxy polymers, so as to accelerate curing reaction, improve wear resistance and mechanical properties, and reduce chemical resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
Synthesis of Hydroxyalkyl urethane Modifier HUM-1
[0083]15.8 g (0.2 AEW) of TMD and 20.4 g (0.2 CCEW) of PC, ratio 1:1, were put into a 100 ml flask and then the mixture was stirred. The reaction mixture was kept in the flask at room temperature during 6 hours and the consumption of the cyclic carbonate groups was controlled by spectrometer FT / IR, (wavelength 1800 cm−1).
[0084]Calculated molecular mass of HUM-1 was 362.
[0085]Solids content (60°, 110° C.) was 97%.
[0086]Viscosity (25° C.) was 79 Pa·s.
Synthesis of Hydroxyalkyl Urethane Modifier HUM-2
[0087]17.0 g (0.2 AEW) of IPDA and 22.4 g (0.22 CCEW) of PC, ratio 1:1.1, were loaded into a 100 ml flask, and then the mixture was stirred. The reaction mixture was kept in the flask at room temperature during 6 hours, and the consumption of the cyclic carbonate groups was controlled by spectrometer FT / IR, (wavelength 1800 cm−1).
[0088]Calculated molecular mass of HUM-2 was 374.
[0089]Solids content (60°, 110° C.) was 96%.
[0090]Viscosity (50° ...
example 1
Composition 1
[0105]18.7 g (0.1 EEW) of DER-331
[0106]0.9 g (5% to epoxy resin) HUM-1
[0107]The mixture was put into a 50 ml vessel, was stirred and heated at 50° C. for 30 min.
[0108]Then the mixture was cooled to RT, 4.0 g (0.1 AHEW) of TMD was added, and the mixture was repeatedly stirred for 2 minutes.
[0109]Then the mixture was poured into standard moulds.
Composition 2
[0110]18.7 g (0.1 EEW) of DER-331
[0111]1.9 g (10% to epoxy resin) HUM-1
[0112]The mixture was placed into a 50 ml vessel, was stirred and heated at 50° C. for 30 min. Then the mixture was cooled to RT, 4.0 g (0.1 AHEW) of TMD was added, and the mixture was repeatedly stirred for 2 minutes.
[0113]Then the mixture was loaded into standard moulds.
Composition 3
[0114]18.7 g (0.1 EEW) of DER-331
[0115]2.8 g (15% to epoxy resin) HUM-1
[0116]The mixture was placed into a 50 ml vessel, and stirred and heated at 50° C. for 30 min.
[0117]Then the mixture was cooled to RT, 4.0 g (0.1 AHEW) of TMD was added, and the mixture was repeated...
example 2
Preparation of epoxy-amine composition—ST-3000, TMD, HUM-2
[0140]Comparative Composition 2 was used in this EXAMPLE.
[0141]The mixture was put into a 50 ml vessel and was stirred for 2 minutes.
[0142]Then the mixture was poured into standard moulds.
Composition 6
[0143]22.5 g (0.1 EEW) ST-3000
[0144]1.1 g (5.0% to epoxy resin) HUM-2
[0145]The mixture was put into a 50 ml vessel, was stirred and heated at 50° C. during 30 min
[0146]Then the mixture was cooled to RT, 4.0 g (0.1 AHEW) of TMD was added, and the mixture was repeatedly stirred for 2 minutes.
[0147]Then the mixture was applied to standard moulds.
Composition 7
[0148]22.5 g (0.1 EEW) ST-3000
[0149]2.2 g (10% to epoxy resin) HUM-2
[0150]The mixture was put into a 50 ml vessel, was stirred and heated at 50° C. during 30 min.
[0151]Then the mixture was cooled to RT, 4.0 g (0.1 AHEW) of TMD was added, and the mixture was repeatedly stirred for 2 minutes.
[0152]Then the mixture was placed into standard moulds.
Composition 8
[0153]22.5 g (0.1 EEW...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com