Chimeric flavivirus vaccines
a technology of chimeric flavivirus and vaccine, applied in the field of attenuated viruses and infectious diseases, can solve the problems of japanese encephalitis, current or potential threats to global public health of several members of the flavivirus family,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
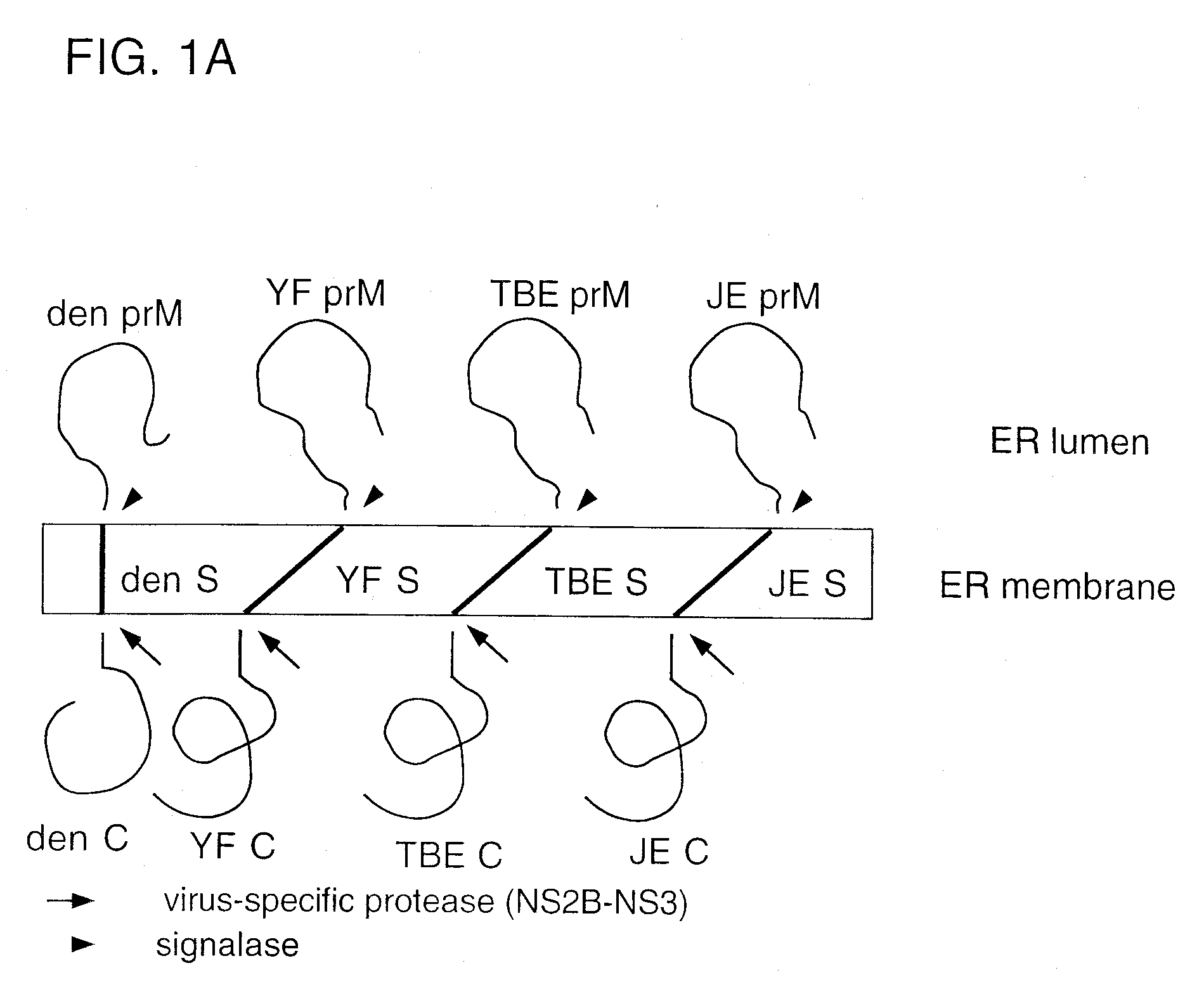
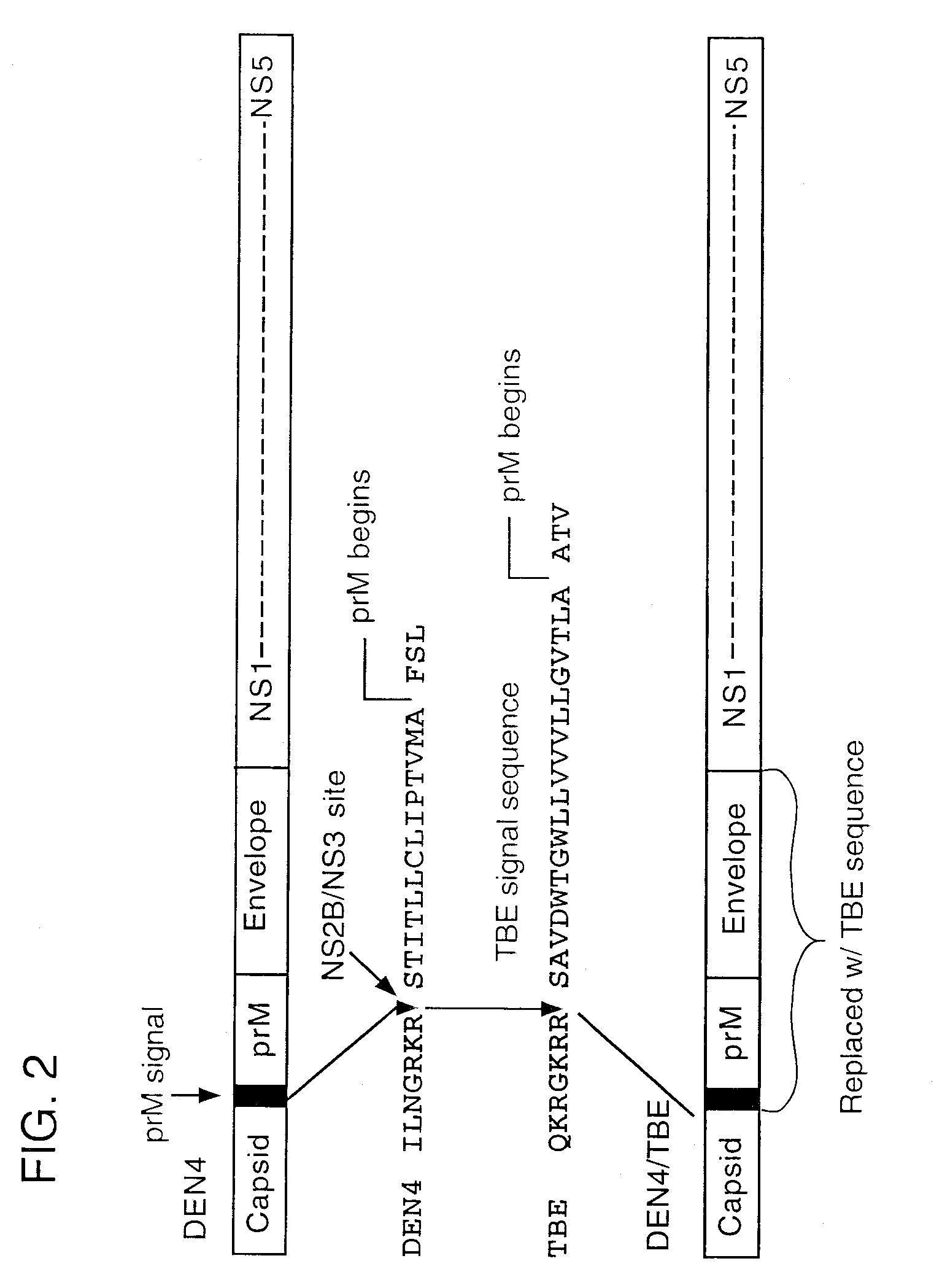
[0056]The invention provides chimeric flaviviruses that can be used in vaccination methods against flavivirus infection. Construction and analysis of chimeric flaviviruses of the invention, such as chimeras of yellow fever virus and Japanese Encephalitis (JE), Dengue types 1-4 (DEN 1-4), Murray Valley Encephalitis (MVE), St. Louis Encephalitis (SLE), West Nile (WN), Tick-borne Encephalitis (TBE), and Hepatitis C (HCV) viruses are described as follows.
[0057]Yellow fever (YF) virus is a member of the Flaviviridae family of small, enveloped positive-strand RNA viruses. Flavivirus proteins are produced by translation of a single long open reading frame to generate a polyprotein, and a complex series of post-translational proteolytic cleavages of the polyprotein by a combination of host and viral proteases, to generate mature viral proteins (Amberg et al., J. Virol. 73:8083-8094, 1999; Fields, “Flaviviridae,” In Virology, Fields (ed.), Raven-Lippincott, New York, 1995, Volume I, p. 937)....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com