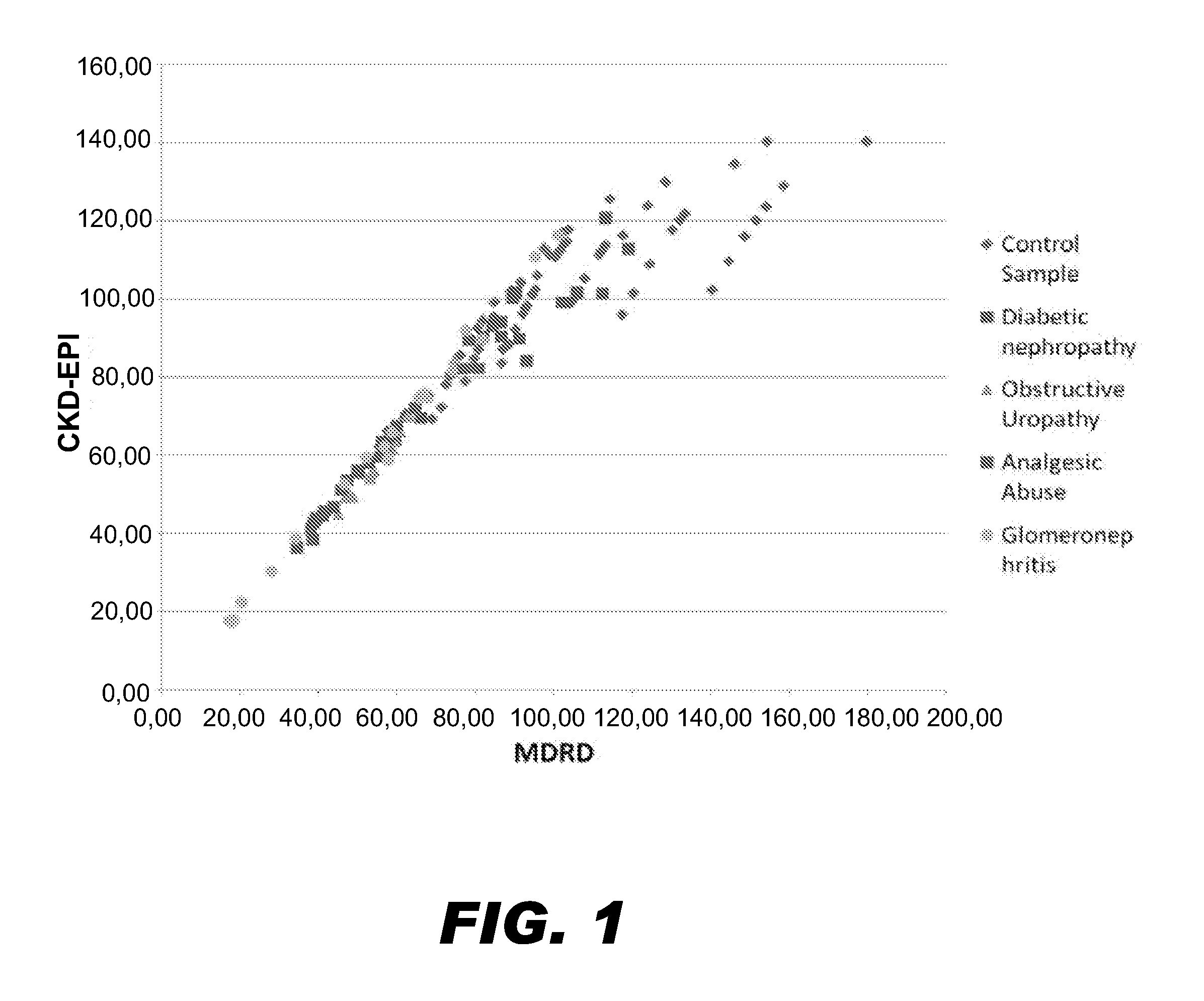
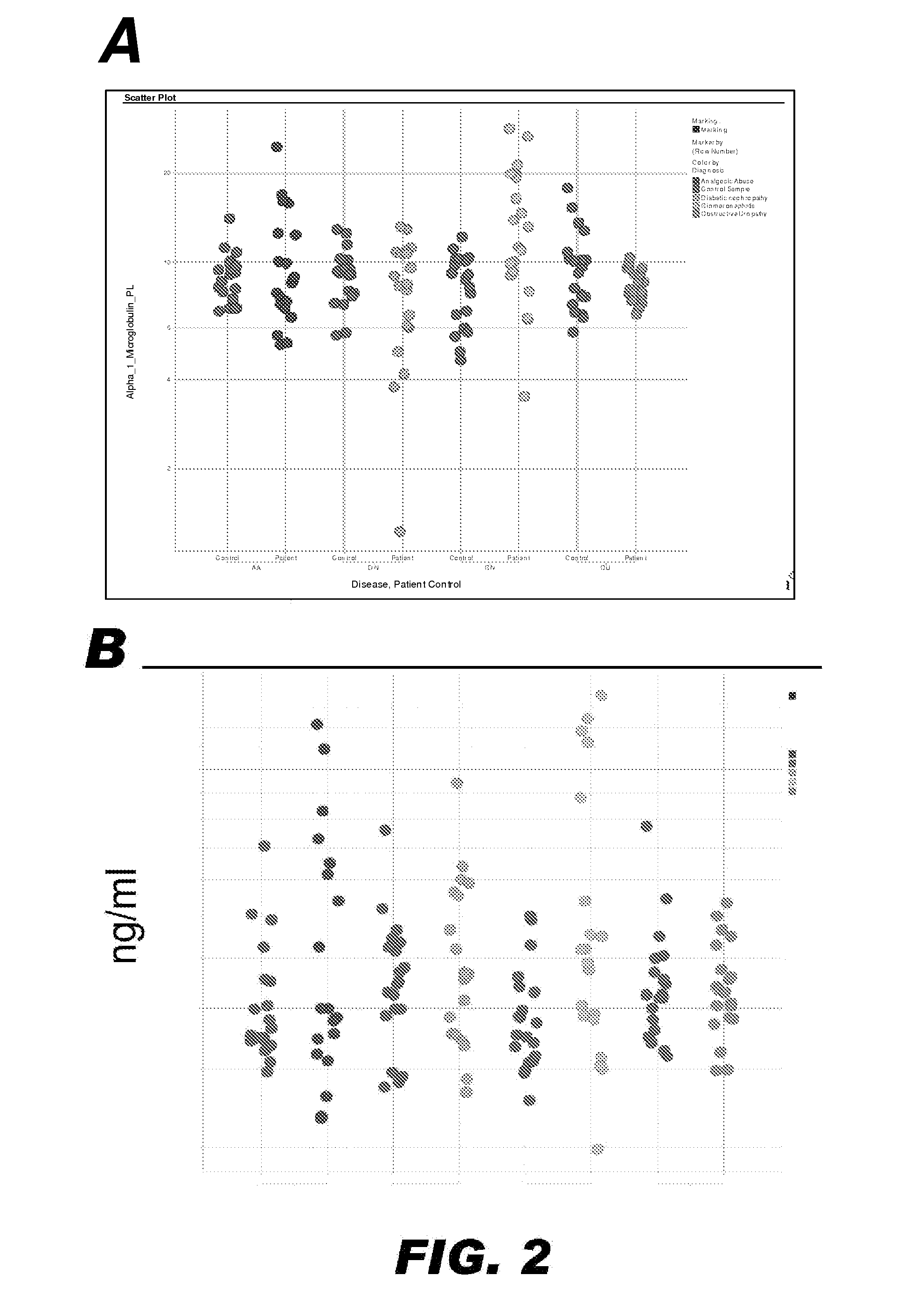
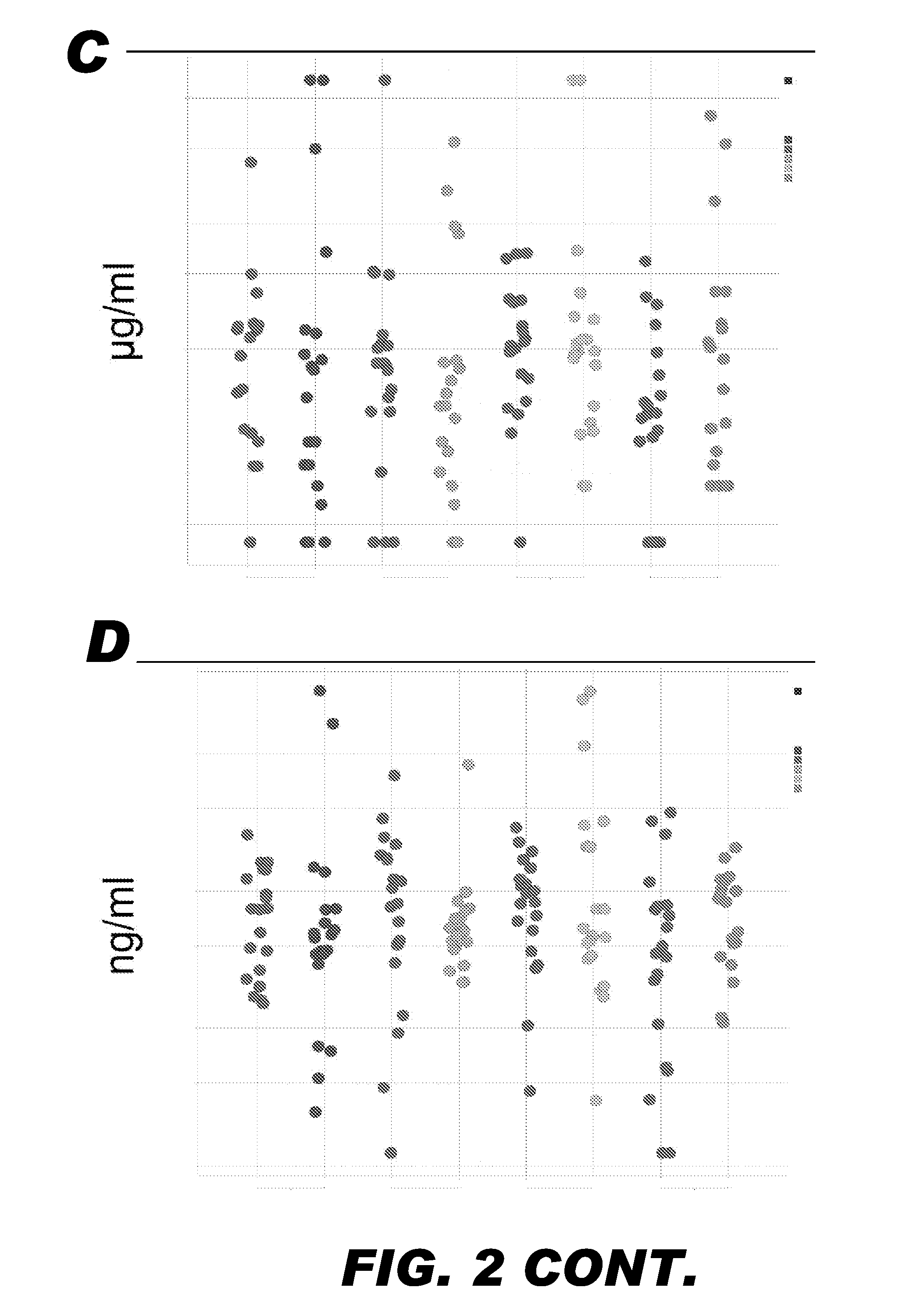
Methods and Devices for Detecting Glomerulonephritis and Associated Disorders
a glomerulonephritis and associated disorder technology, applied in the field of methods and devices for diagnosing, monitoring or determining glomerulonephritis or an associated disorder in a mammal, can solve the problems of inability to detect the disease, the kidney is especially vulnerable to injury, and the test is also generally costly, slow, and/or invasiv
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Least Detectable Dose and Lower Limit of Quantitation of Assay for Analytes Associated with Renal Disorders
[0093]To assess the least detectable doses (LDD) and lower limits of quantitation (LLOQ) of a variety of analytes associated with renal disorders, the following experiment was conducted. The analytes measured were alpha-1 microglobulin (A1M), beta-2 microglobulin (B2M), calbindin, clusterin, CTGF, cystatin C, GST-alpha, KIM-1, NGAL, osteopontin (OPN), THP, TIMP-1, TFF-3, and VEGF.
[0094]The concentrations of the analytes were measured using a capture-sandwich assay using antigen-specific antibodies. For each analyte, a range of standard sample dilutions ranging over about four orders of magnitude of analyte concentration were measured using the assay in order to obtain data used to construct a standard dose response curve. The dynamic range for each of the analytes, defined herein as the range of analyte concentrations measured to determine its dose response curve, is presented ...
example 2
Precision of Assay for Analytes Associated with Renal Disorders
[0101]To assess the precision of an assay used to measure the concentration of analytes associated with renal disorders, the following experiment was conducted. The analytes measured were alpha-1 microglobulin (A1M), beta-2 microglobulin (B2M), calbindin, clusterin, CTGF, cystatin C, GST-alpha, KIM-1, NGAL, osteopontin (OPN), THP, TIMP-1, TFF-3, and VEGF. For each analyte, three concentration levels of standard solution were measured in triplicate during three runs using the methods described in Example 1. The percent errors for each run at each concentration are presented in Table 3 for all of the analytes tested:
TABLE 3Precision of Analyte AssayAverageRun 1Run 2Run 2InterrunconcentrationErrorErrorErrorErrorAnalyte(ng / mL)(%)(%)(%)(%)Calbindin4.0626133653272811603Clusterin4.449263951682291302CTGF1.210174142.5191914141875139GST-alpha3.91475101613710114211668KIM-10.035205130.3245282.90574VEGF65101614534921275,397113149β-2 ...
example 3
Linearity of Assay for Analytes Associated with Renal Disorders
[0103]To assess the linearity of an assay used to measure the concentration of analytes associated with renal disorders, the following experiment was conducted. The analytes measured were alpha-1 microglobulin (A1M), beta-2 microglobulin (B2M), calbindin, clusterin, CTGF, cystatin C, GST-alpha, KIM-1, NGAL, osteopontin (OPN), THP, TIMP-1, TFF-3, and VEGF. For each analyte, three concentration levels of standard solution were measured in triplicate during three runs using the methods described in Example 1. Linearity of the assay used to measure each analyte was determined by measuring the concentrations of standard samples that were serially-diluted throughout the assay range. The % recovery was calculated as observed vs. expected concentration based on the dose-response curve. The results of the linearity analysis are summarized in Table 4.
TABLE 4Linearity of Analyte AssayExpectedObservedRecoveryAnalyteDilutionconcentra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com