Protein vaccines against poxviruses
a technology of poxvirus and protein, applied in the field of protein vaccines against poxviruses, can solve the problems of protein alone not conferring acceptable protection, the vaccine that eradicates pox worldwide poses serious side effects in a subset of people with acquired or congenital defects in the immune system, and the monkeypox cannot be eradicated. , to achieve the effect of increasing cross-reactivity, cross-protection, and high degree of cross-reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
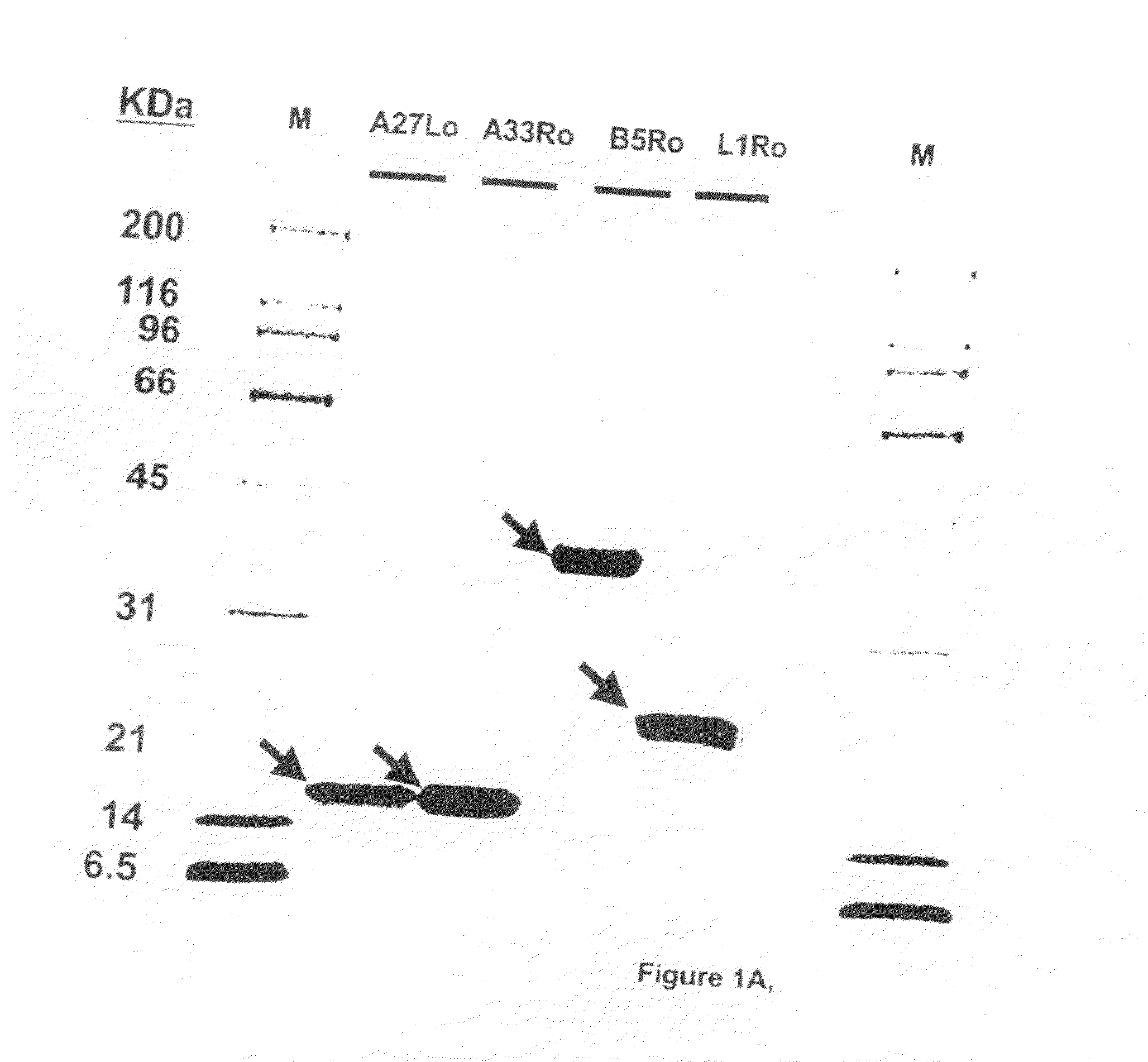
[0089]In this paper is disclosed novel protein vaccines and methods of vaccination against poxviruses which contains monkeypox ortholog proteins or corresponding ortholog proteins of vaccinia or monkeypox. It is now possible to use such exogenously expressed proteins, or portions of proteins, to elicit immune responses that contribute to the protection against poxviruses in humans and other mammals. As described above, the novel protein vaccine includes at least two of the following purified recombinant monkeypox virus protein or peptide products produced by the monkeypox virus M1R, A35R, A29L and B6R open reading frames, and orthologs of these proteins or peptides having 90% amino acid identity, and an adjuvant. At least one of the peptides / proteins should be specific to an IMV immunogen (ortholog product of M1R or A29L) and at least one should be specific to an EEV immunogen (ortholog product of A35R or B6R). As noted above, preferably such orthologs are obtained from an orthopoxv...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com