Method for identification of protease activity inhibitors and assaying the presence of protease activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Expression of pBD-NFκB in Mammalian Cells
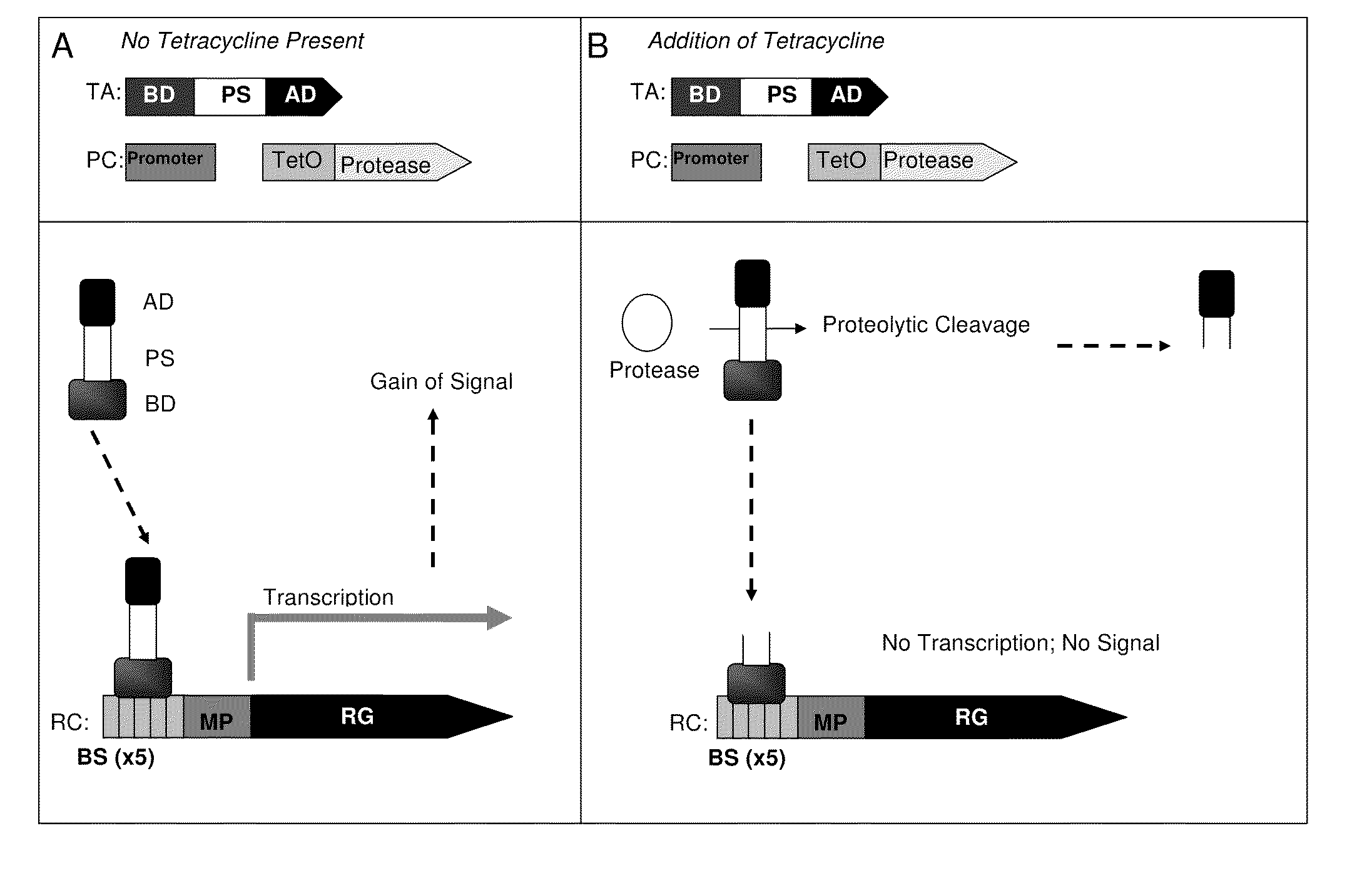
[0055]A RC in accordance with one embodiment of the present invention, containing a synthetic promoter G5 / TO4 was transfected by a lentiviral vector into cells. The RC was co-transfected with a plasmid expressing a Gal4 binding domain fused to the NFκB activator fusion (pBD-NFκB) (the TA agent) into HeLa-tTS cells. The transfected cells constitutively express the tetracycline transcription silencer tTS (Clontech). The cells were grown in a 6-well plate to approximately 80% confluence. Six hours after transfection, 1 ug / ml of tetracycline was added to the media of the test cells. Tetracycline was not added to the media of cells used as a control. Culture medium was collected two days post-transfection for the Gaussia luciferase (GLuc) assay. Monique Verhaegen and Theodore K. Christopoulos Anal. Chem., 74:4378-4385 (2002). Venus expression was observed with a fluorescent microscope. The relative light units (RLU) of the cells cultured in medium...
example 2
tTS Cells Comprising the RC and TA Agent
[0058]A gene for the regulatory component, a transactivator chimeric fusion protein consisting of the appropriate BoNT substrate, SNAP-25 for BoNT / A and VAMP-2 for BoNT / B, sandwiched between a Gal4 DNA binding domain (amino acids 1-148) (Gal4 / BD or BD) and the NFκB transactivation domain (NFκB / AD or AD) was transduced into the cells that have the novel Reporter construct as described in Example 1.
example 2a
[0059]The BD-PS-AD constructs, in which the protein substrate does not contain palmitoylated residues, were constructed synthetically and introduced in cells containing the RC. The TA agent encoding either 103 amino acid residues around the cleavage site of SNAP-25 (residues 104-206) or 70 amino acid residues around the cleavage site of VAMP-2 (residues 25-94) fused between the Gal4 DNA binding domain and the NFκB transactivator domain were used. The reporter cell line clone #17 from Example 1 was further transduced with a lentiviral vector that carries the BD-VAMP-NFκB transactivator gene construct. Six single cell clones were selected and analyzed for the ratio of bioluminescence in the presence and absence of tetracycline. See FIG. 7. The transduced cells were subjected to appropriate selection (G418, blasticidin, and puromycin), and single-cell clones carrying stable integrations of both the reporter and the VAMP-2 transactivator fusion were obtained. The reporter gene in clone ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com