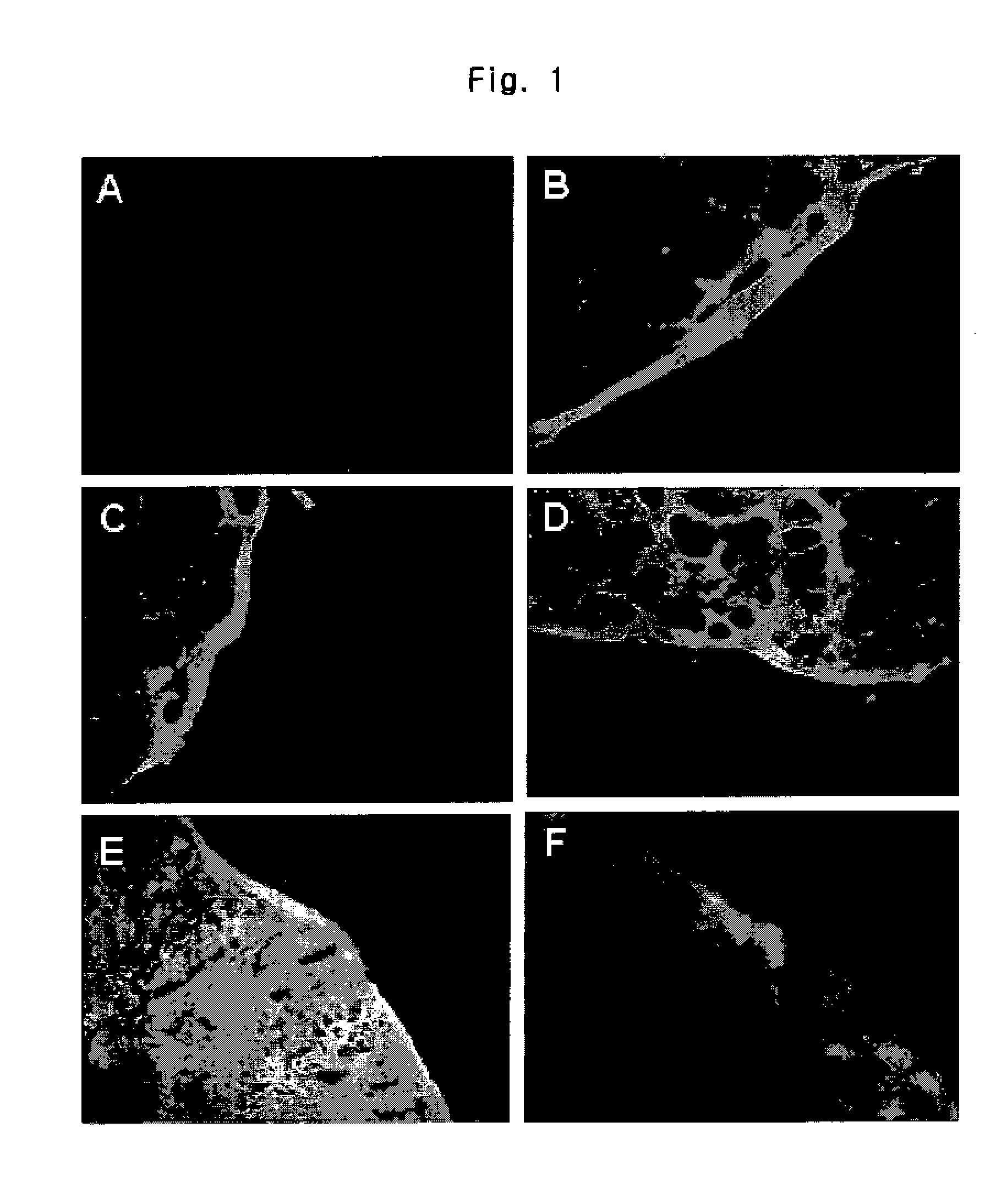
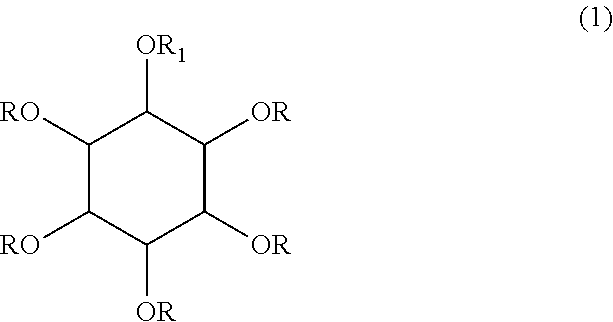
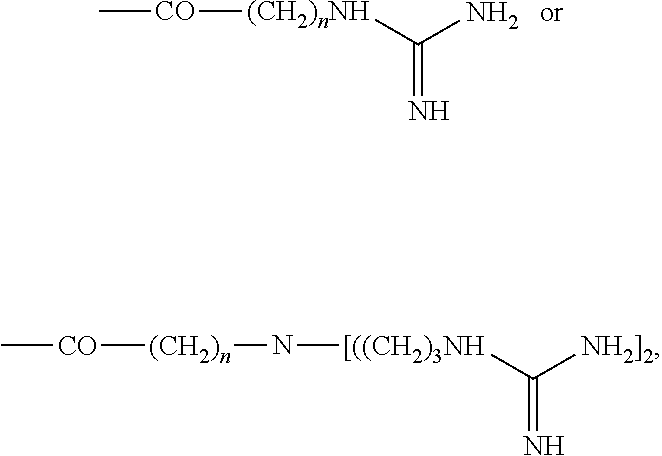
Inositol and trehalose derivatives and pharmaceutical compositions for treating neurodegenerative diseases comprising the same
a technology which is applied in the field of inositol and trehalose derivatives, can solve the problems of inability to cure the disease, inositol is known to have difficulty in passing through the blood-brain barrier (bbb), and the agent cannot cure the disease, so as to improve the permeability of the bbb
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example
Preparative Example 1
Preparation of Scyllo-Inositol Having Carbobenzoxy (Cbz)-Protected Linker
Introduction of Acetonide Protecting Group
[0074]
[0075]1-O-Bz-2,3,4,5,6-pentahydroxyl-scyllo-inositol (Korean Patent No. 578732; 9.96 g, 35.05 mmol) was dissolved in N,N-dimethylformamide (110 ml), and para-toluene sulfonic acid (3.33 g) was added dropwise thereto to obtain a mixture. 2-Methoxypropene (33.5 ml) was added to the mixture and the mixture was stirred at room temperature for 16 hours.
[0076]After the completion of the reaction, the reaction mixture was extracted with ethyl acetate, and the extract was washed several times with saturated aqueous NaHCO3 and water. The organic layer thus obtained was dried over Na2SO4, concentrated under a reduced pressure, and then purified by column chromatography (ethyl acetate:hexane=1:2) to obtain the title compound having two isopropylidene groups introduced to the backbone thereof, as a white foamy solid (6.03 g).
[0077]1H NMR (CDCl3): δ 1.44-...
example 1
Preparation of Scyllo-Inositol Derivative Having Ten Guanidine Groups
[0135] Introduction of Side Chain to scyllo-Inositol by Acylation
[0136]The compound having five OH groups obtained in Preparative Example (34 mg, 0.079 mmol), a branched linker having two guanidine groups (Korean Patent No. 699279; 464 mg, 0.636 mmol), 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (151 mg, 0.79 mmol) and 4-dimethylaminopyridine (24 mg, 0.2 mmol) were dissolved in N,N-dimethylformamide (2 ml), and stirred at room temperature for two days.
[0137]After the completion of the reaction, the reaction mixture was washed several times with water and aqueous NaHCO3. The organic layer thus obtained was dried over Na2SO4, concentrated under a reduced pressure, and then purified by column chromatography (dichloromethane:methanol=10:1) to obtain the title compound having five acyl side chains, as a sticky solid (228 mg).
[0138]1H NMR (CDCl3): δ 1.25-1.69 (m, 236H), 2.16-2.25 (m, 12H), 2.30-2.46 (m, 30H), 3.14 (m,...
example 2
Preparation of Scyllo-Inositol Derivative Having Five Guanidine Groups
[0148] Introduction of Side Chain to scyllo-Inositol by Acylation
[0149]The compound having five OH groups obtained in Preparative Example (85.8 mg, 0.2 mmol), a linear linker having a single guanidine group (600 mg, 1.6 mmol), 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (383.42 mg, 2 mmol) and 4-dimethylaminopyridine (61 mg, 0.5 mmol) were dissolved in N,N-dimethylformamide (2 ml) and stirred at room temperature for two days.
[0150]After the completion of the reaction, the reaction mixture was washed several times with water and aqueous NaHCO3. The organic layer thus obtained was dried over Na2SO4, concentrated under a reduced pressure, and then purified by column chromatography (dichloromethane:methanol=10:1) to obtain the title compound having five acyl side chains, as a sticky solid (402 mg).
[0151]1H NMR (CDCl3, 500 MHz): δ 1.28-1.38 (m, 12H), 1.47-1.62 (m, 114H), 2.22-2.42 (m, 12H), 3.18-3.21 (m, 2H), 3.37-3...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com