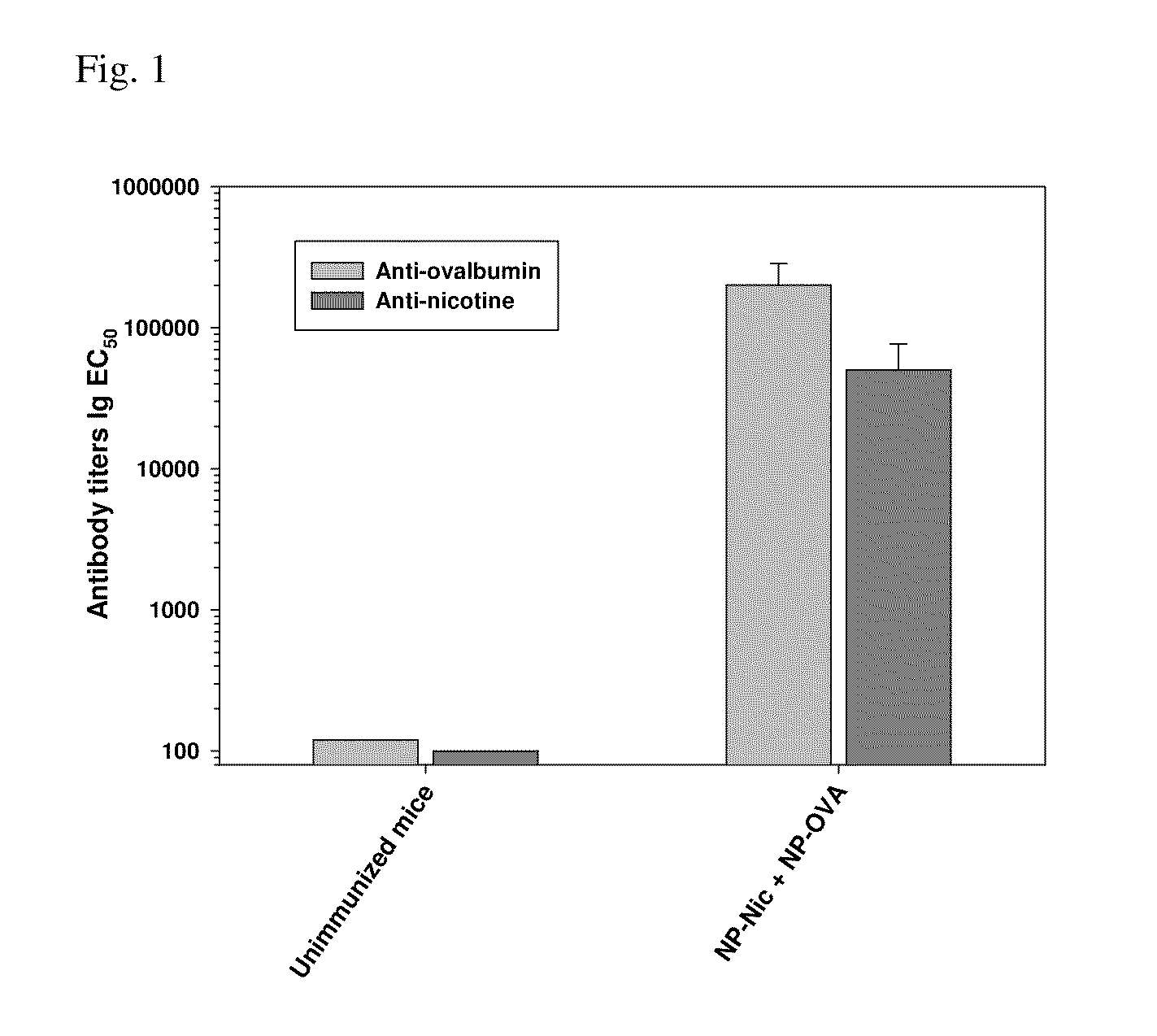
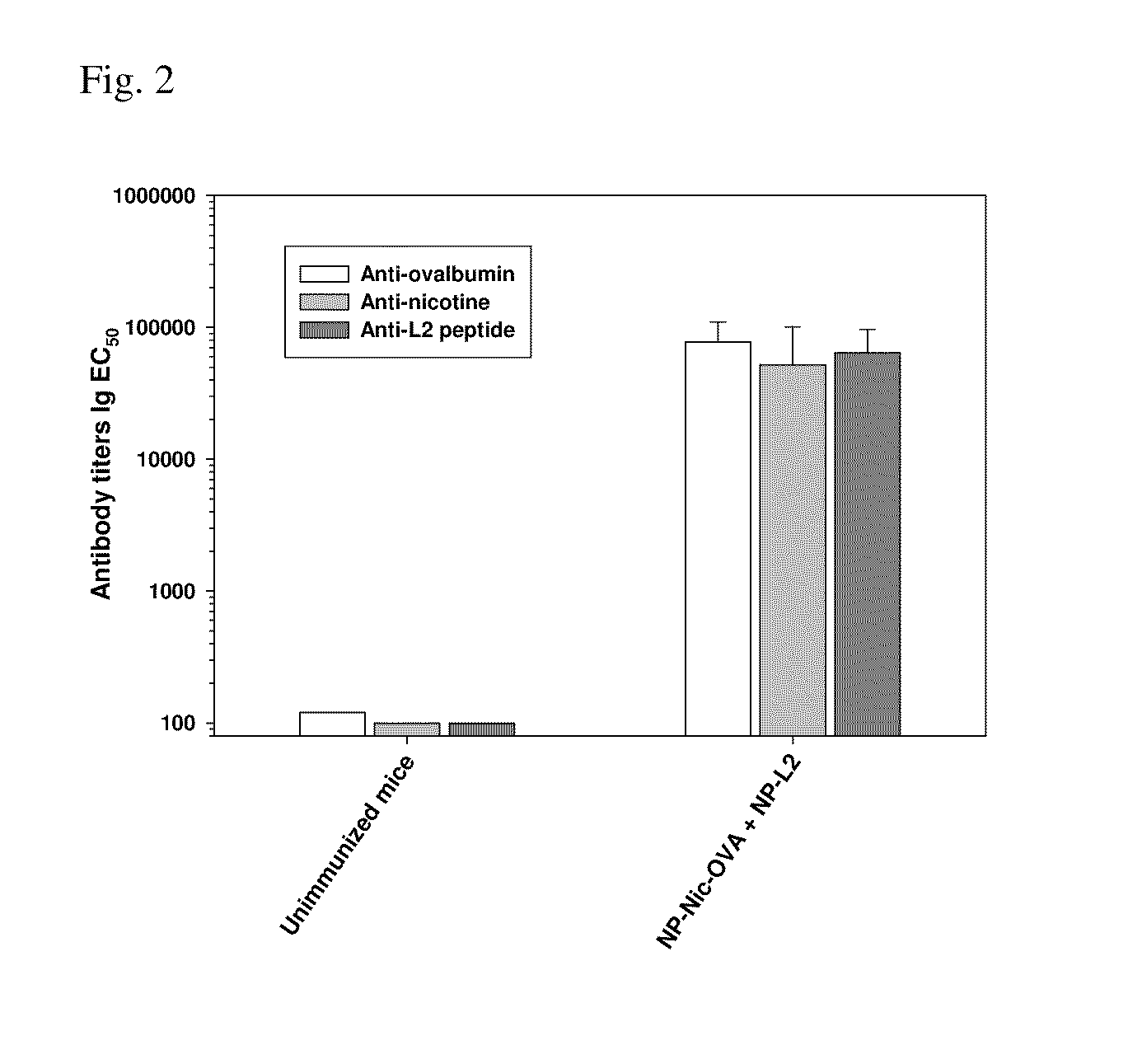
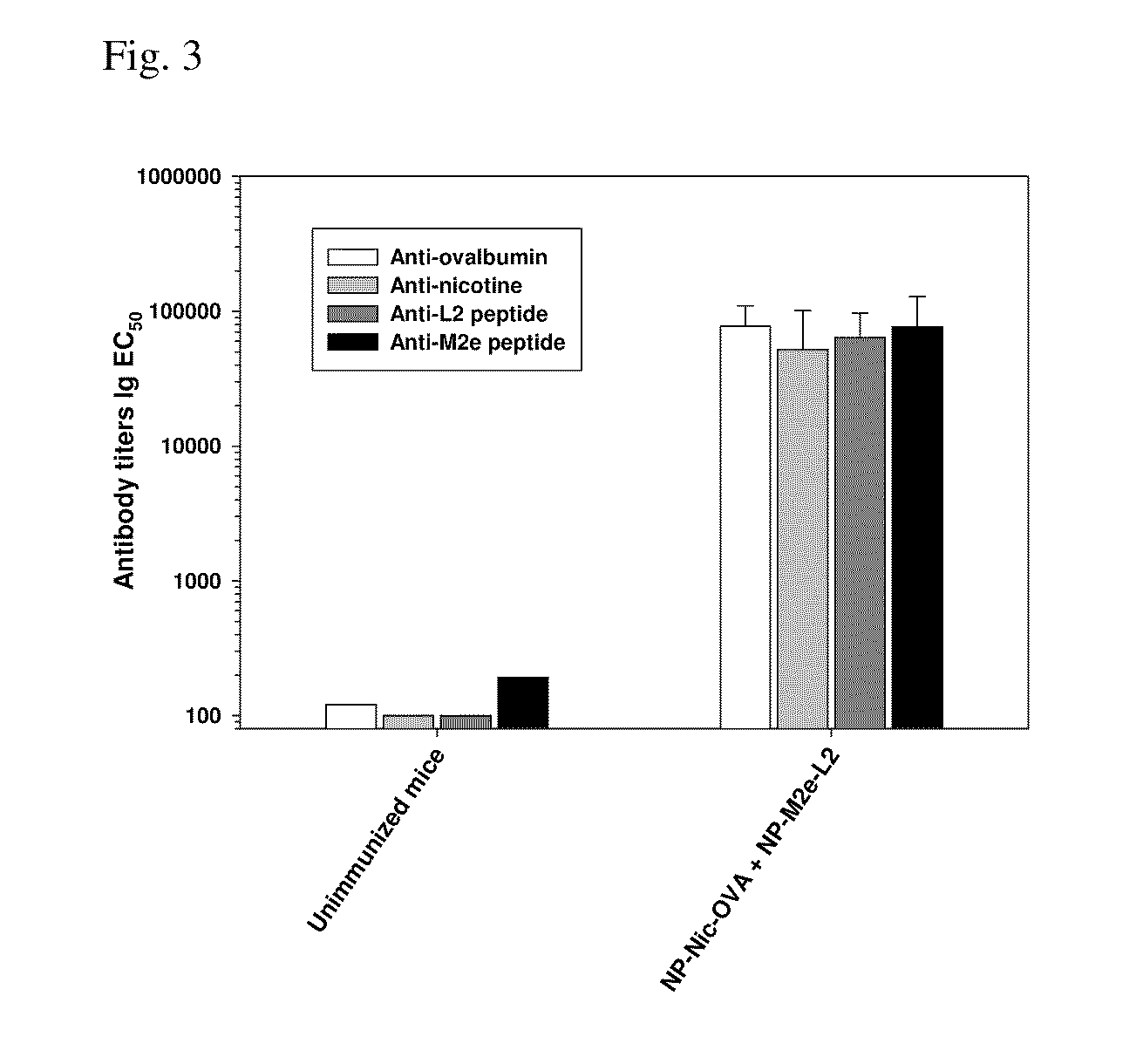
Multivalent synthetic nanocarrier vaccines
a vaccine and synthetic technology, applied in the field of multivalent synthetic nanocarrier vaccines, can solve the problems of low yield, complex conjugation of antigens to protein carriers, and current multivalent vaccines and methods that need improvemen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Formulation for First Population of Nanocarriers (Prophetic)
[0215]Synthetic nanocarriers comprising PLGA-R848 conjugate (adjuvant), PLA-PEG-N3 conjugate (linker to peptide antigen) and ova peptide (T-cell antigen) are prepared via a double emulsion method wherein the ova peptide is encapsulated in the synthetic nanocarriers. To a suspension of the synthetic nanocarriers (10 mg / mL in PBS (pH 7.4 buffer), 5 mL, containing about 12.5 mg (MW: 20, 000; 0.000625 mmol) of PLA-PEG-N3) is added HPV L1-peptide comprising an acetylene linker (33 mg) under gentle stirring. A solution of sodium ascorbate (100 mM in H2O, 0.3 mL) is added, followed by CuSO4 solution (10 mM in water, 0.6 mL). The resulting light yellow suspension is stirred at 20 C for 15 h and additional CuSO4 solution (0.3 mL) and sodium ascorbate solution (0.15 mL) are added. The suspension is stirred for 5 h at 20 C and diluted with PBS buffer (pH 7.4) to 10 mL and is centrifuged to remove the supernatant. The residual nanocarr...
example 2
Formulation for Second Population of Nanocarriers (Prophetic)
[0216]Using the general procedures outlines in Example 1 above, synthetic nanocarriers comprising PLA-R848, PLA-PEG-N3 and encapsulated ova peptide are prepared and conjugated with an HPV L2 peptide to provide L2 peptide conjugated synthetic nanocarriers.
example 3
Formulation Combining First and Second Populations of Nanocarriers (Prophetic)
[0217]The synthetic nanocarrier preparations from Examples 1 and 2 above are thawed and diluted in PBS to a final concentration of 5 mg of nanocarriers per milliliter. Equal aliquots of each (0.5 mL) are combined to provide a population of nanocarriers that contain both the HPV L1 and L2 peptides.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com