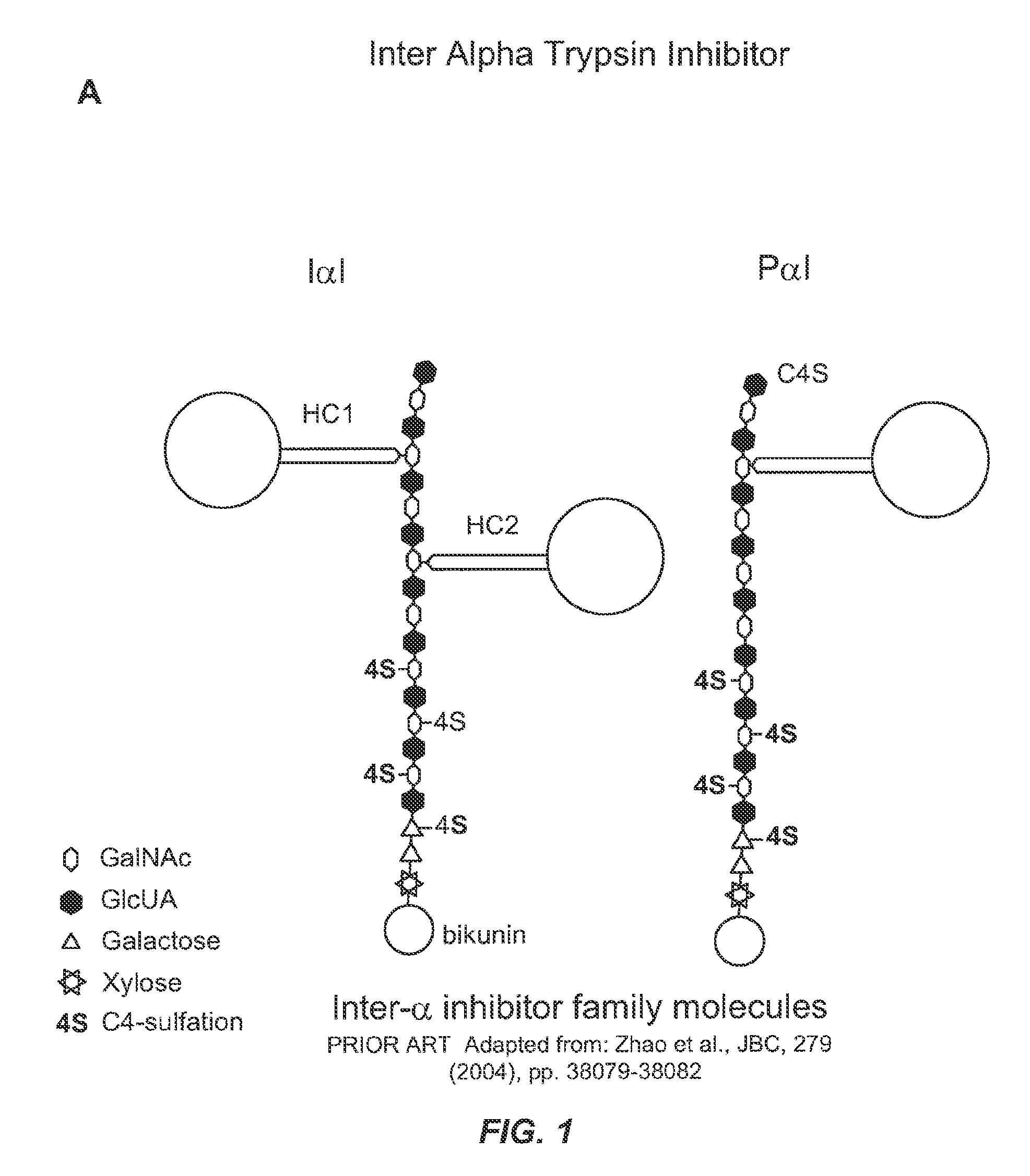
MANUFACTURE OF INTER-ALPHA-INHIBITOR (IaIp) FROM PLASMA
a technology of interalpha-inhibitor and plasma, which is applied in the field of plasma production, can solve the problems of limited commercially available blood products, inability to increase the supply of these products, and inability to manufacture new plasma raw, etc., and achieve the effect of promoting epithelial repair
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
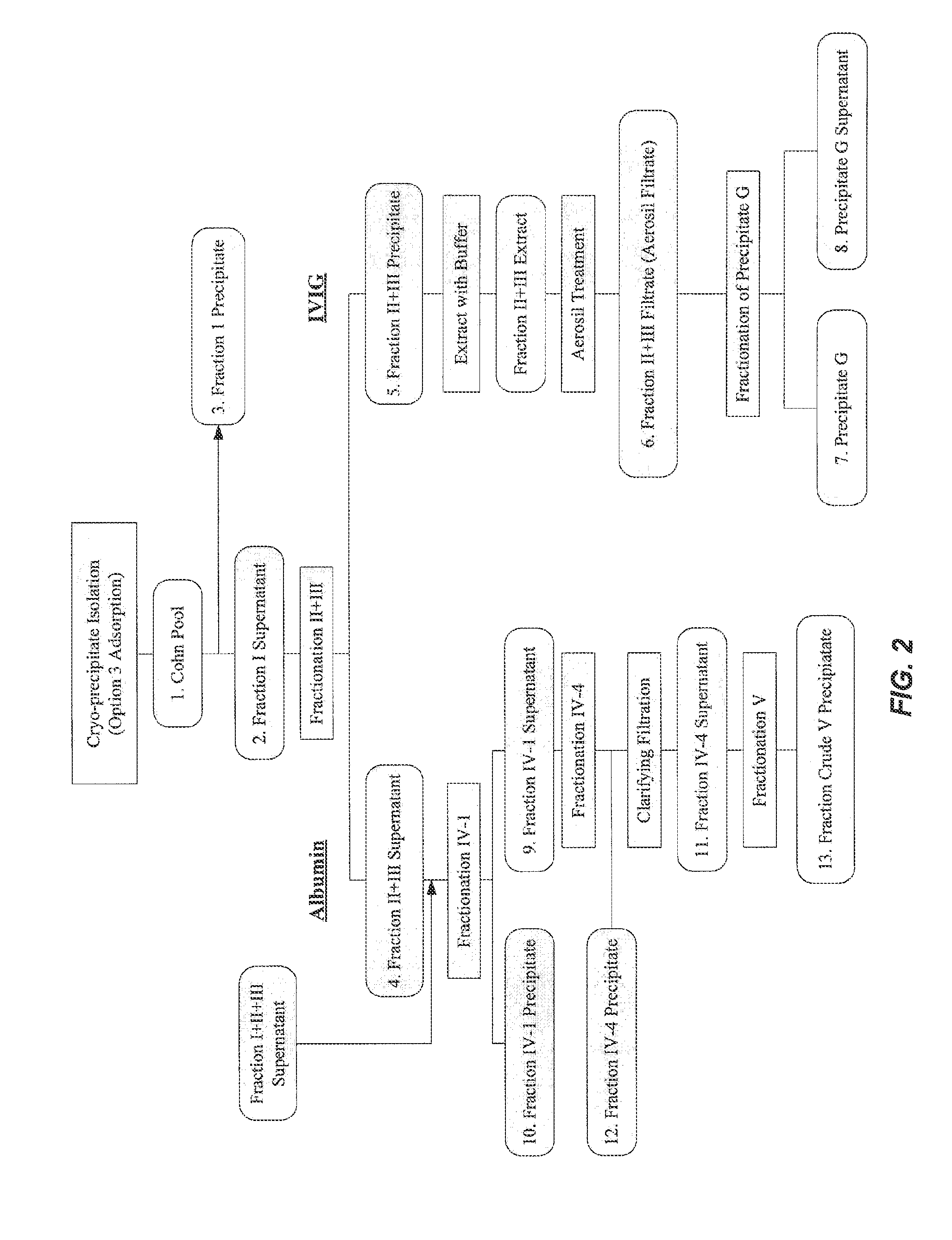
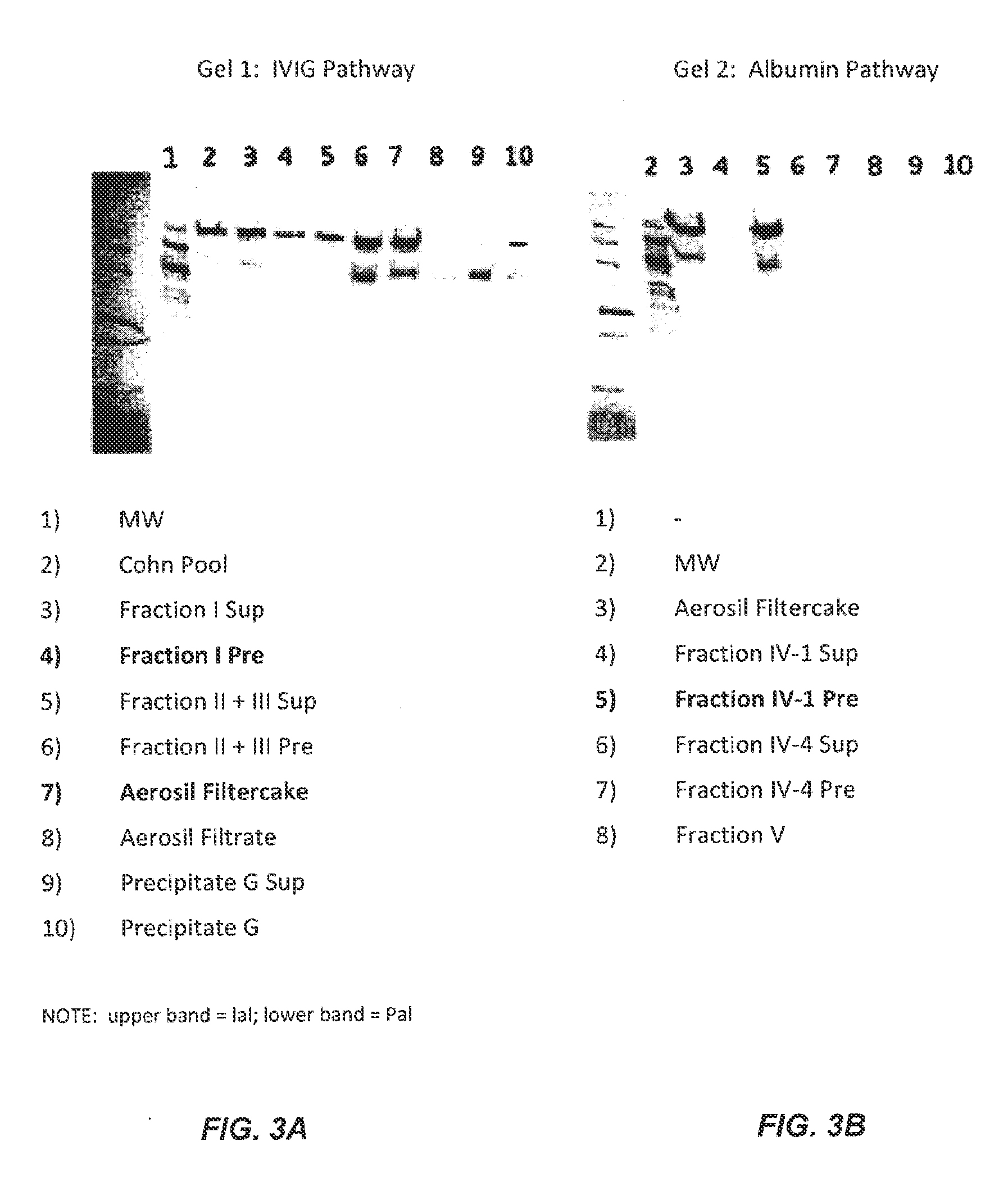
[0430]To determine an economically beneficial scheme for the manufacture of Inter-alpha-Inhibitor (IaIp) from a plasma sample, which allows for the recovery of additional blood factors from the same plasma sample, a lot of pooled human plasma was subjected to industrial fractionation according to the scheme outlined in the flow-diagram shown in FIG. 2. The fate of IaIp in the industrial fractionation process was followed by Western blot using an antibody specific for the small subunit, bikunin. Due to the size difference between IaI and PaI, the anti-bikunin antibody allowed for the identification of both proteins in the fractionation process, which were distinguished based on their migration on the SDS-PAGE gel (FIG. 3).
[0431]As seen in FIG. 3, a majority of the IaIp present in the pooled plasma sample was fractionated into three major fractions, the Fraction I precipitate, the Fraction II+III precipitate filter cake, and the Fraction IV-1 precipitate. Advantageously, all three of ...
example 2
[0432]The present example describes experiments performed to determine the feasibility of extracting IaIp from a Fraction II+III filter cake. Briefly, the Fraction II+III filter cake from the plasma fractionation performed in Example 1 was dissolved in an IaIp extraction buffer (25 mM Tris (pH 8.0); 5 mM EDTA; 200 mM NaCl) at a ratio of 25:1 (mL buffer:g filtercake). The dissolved protein solution was clarified by centrifugation and filtration through a 0.45 μm filter. The conductivity of the resulting suspension was then adjusted by diluting the solution 3:1 with low salt extraction buffer (25 mM Tris (pH 8.0); 5 mM EDTA).
[0433]The clarified Fraction II+III filter cake suspension was then loaded onto a DEAE-Sepharose chromatograph column equilibrated with a low salt buffer (25 mM Tris; 5 mM EDTA; 50 mM NaCl; pH 8.0). A linear gradient from 50 mM NaCl to 500 mM NaCl (25 mM Tris; 5 mM EDTA; NaCl; pH 8.0) was then used to elute the IaIp from the DEAE-Sepharose column, the eluate of wh...
example 3
[0435]In order to aid with the industrial scale-up for the IaIp purification after extraction, an alternate purification scheme was devised that replaces the salt gradient elution of the chromatography columns with a series of step elutions that are more amenable to a large scale manufacturing process. Briefly, an IaIp composition extracted from a Fraction II+III filter cake, as described in Example 2, was loaded onto a DEAE-Sepharose chromatography column equilibrated with a low salt buffer (25 mM Tris; 5 mM EDTA; 65 mM NaCl; pH 8.0). The conductivity of the load was similar to that of the equilibration buffer (about 9 mS / cm). After the load, the column was washed with buffer containing 65 mM NaCl for 5 column volumes (CV) to remove the unbound protein impurities. The flow-through fractions contain very little IaIp as shown by the Western blot results in FIG. 7C.
[0436]In a first step elution, the salt concentration of the buffer (25 mM Tris (pH 8.0); 5 mM EDTA; NaCl) was increased ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com