Polymer Conjugates with Decreased Antigenicity, Methods of Preparation and Uses Thereof
a polymer conjugate and antigenicity technology, applied in the field of protein biochemistry and the pharmaceutical and medical sciences, can solve the problems of increasing the cost of therapy, affecting the maintenance of therapeutically useful concentrations of small recombinant proteins in the circulation, and short half-lives of small proteins following i.v. administration, and achieve the effect of reducing or substantially reducing antigenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation and Testing of Antibodies to MonomethoxyPEG
[0149]It has been reported previously that rabbits can be immunized to various PEGs by immunizing the animals with conjugates in which PEG was coupled to an immunogenic carrier protein (Richter, A. W., et al. (1983) Int Arch Allergy Appl Immunol 70:124-131). A monoclonal antibody that reacts with the polyether backbone of PEG has been developed by injecting mice with an mPEG conjugate of β-glucuronidase and selecting hybridoma clones that produce antibodies to PEG (Cheng, T-L., et al. (1999), supra; Cheng, T L., et al. (2000), supra; Tsai, N.-M., et al. (2001), supra.; Roffler, S., et al., published U.S. Patent Application No. 2001 / 0028881 A1 and U.S. Pat. Nos. 6,596,849 and 6,617,118; the disclosures of all of which are incorporated herein by reference in their entireties). Another monoclonal antibody that reacts with the polyether backbone of PEG has been disclosed recently by Roberts, M. J., et al., in U.S. Patent Application...
example 2
Demonstration of the Role of the Methoxyl Group in the Antigenicity of mPEG
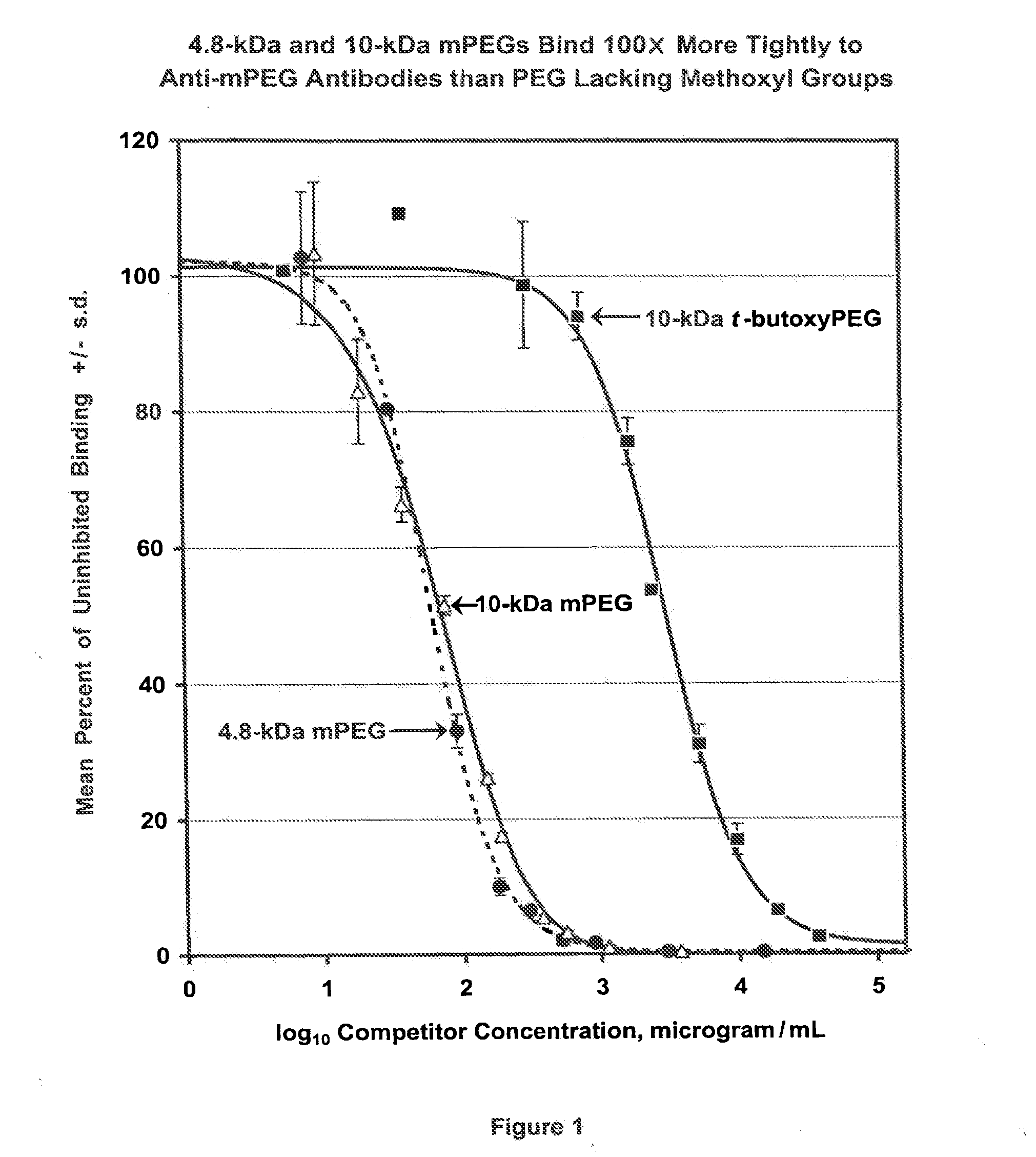
[0153]Unexpectedly, the present inventors have found that the anti-PEG antibodies prepared as described in Example 1 were directed predominantly against the methoxyl end group of the mPEG component of the antigen. FIG. 1 depicts the results from a competitive ELISA assay in which 96-well plates were coated with an mPEG conjugate of a protein that is structurally unrelated to uricase. After blocking the plate with 2% goat serum, solutions of increasing concentration of 4.8-kDa mPEG (Polymer Laboratories, catalog #6570-5010), 10-kDa mPEG (Union Carbide, catalog #MPEG-10,000) or 10-kDa t-butoxyPEG (Polymer Laboratories, catalog #29999997) were added and incubated with a 1:1,000 dilution of rabbit antiserum formed against the mPEG-uricase conjugate. After removing the solution, the extent of anti-PEG antibody bound to the mPEG-protein conjugate on the plate was measured spectrophotometrically, using a peroxidase-...
example 3
Testing of Anti-PEG Antibodies with PEGs Lacking Methyoxyl Groups
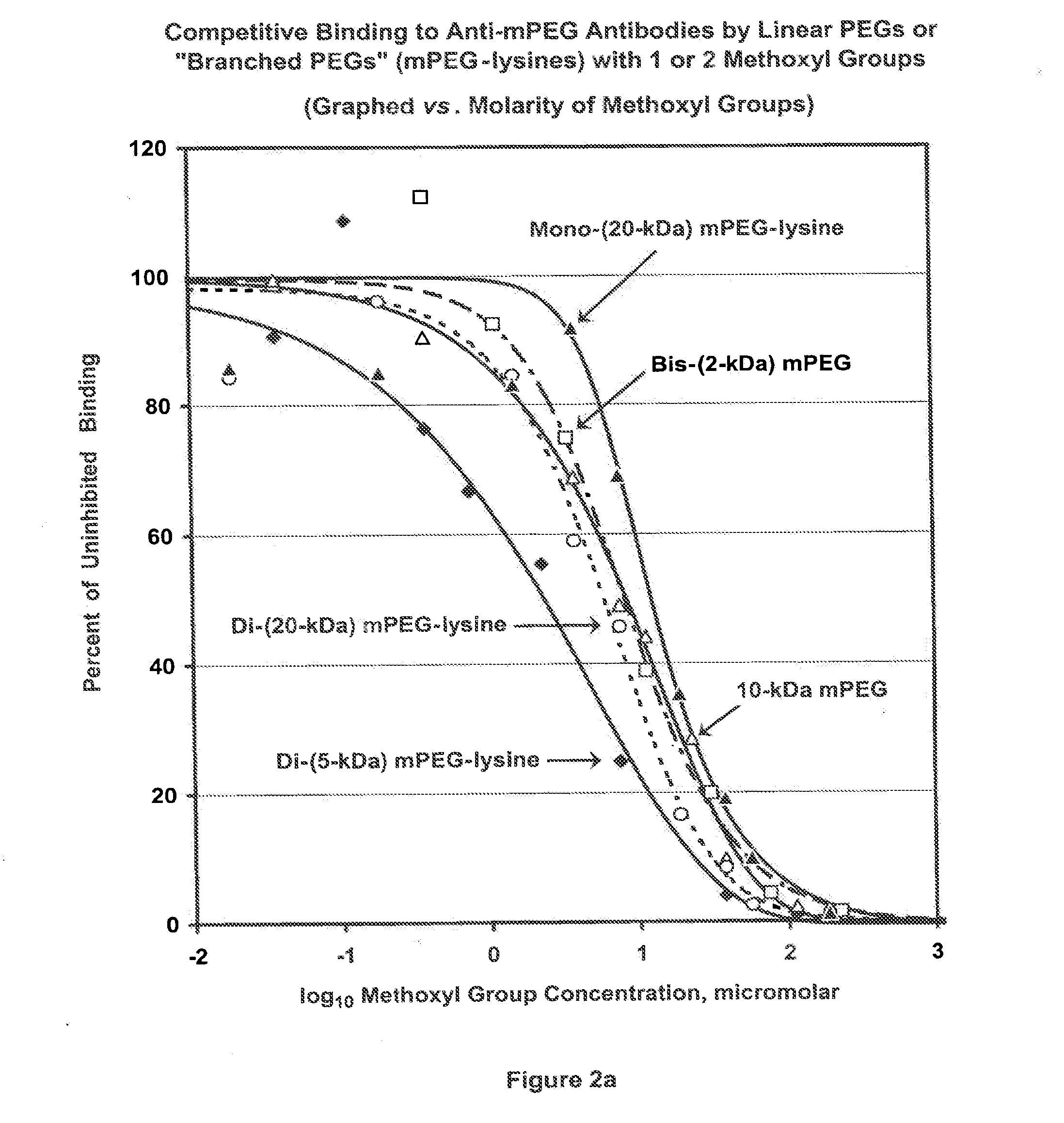
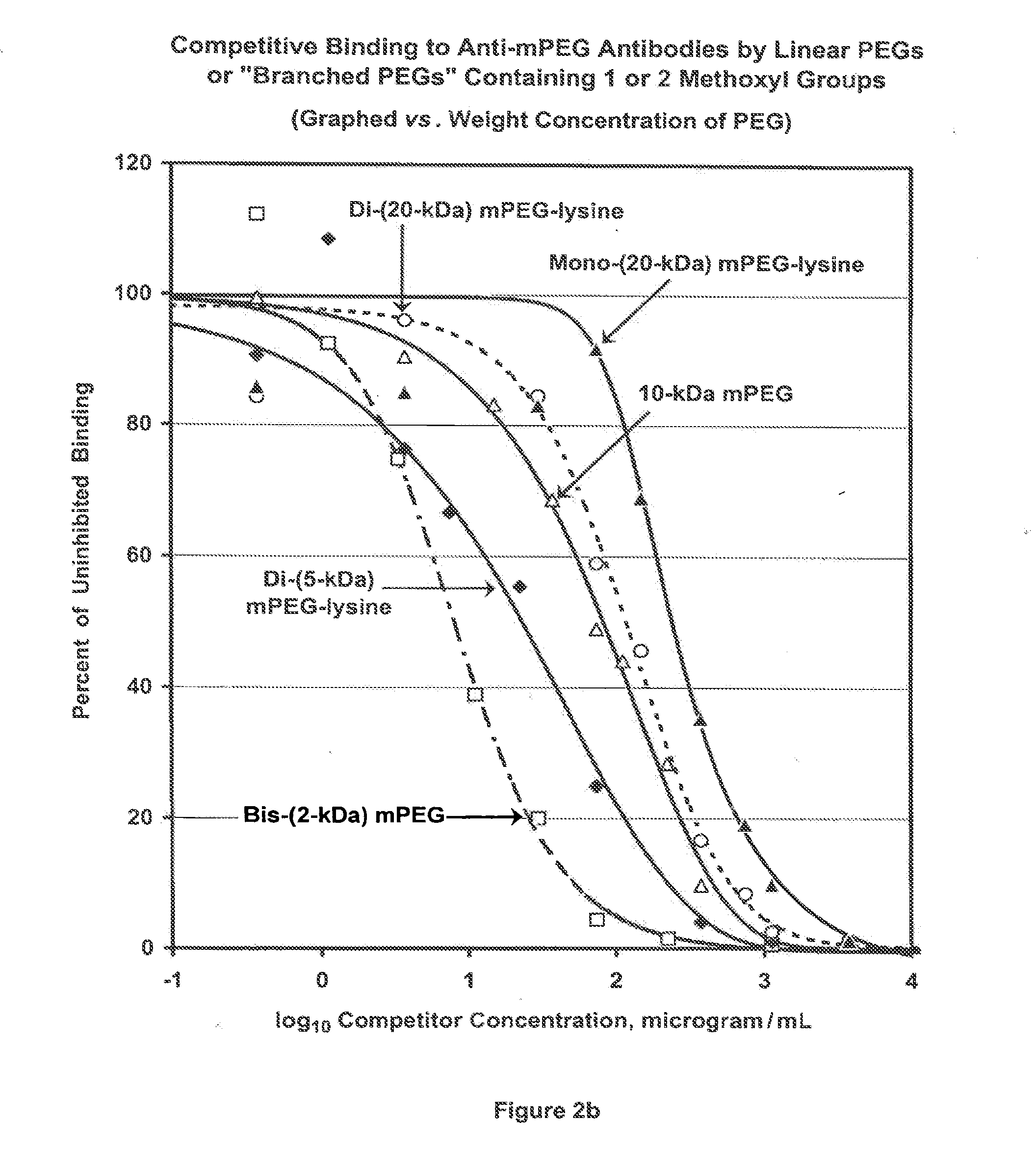
[0158]The term “PharmaPEG” is used herein to refer to linear or branched PEGs lacking an antigenic group at the terminus or termini that is or are distal from the terminus that is activated or can be activated. From the previous examples, it can be inferred that the antigenicity of a polymer, and therefore of a polymer conjugate of a bioactive agent, is a function of the content of methoxyl groups in the polymer. To further test this inference, competitive ELISA analyses were performed, as described for FIG. 1, comparing the abilities of mPEG (“4.8-kDa mPEG”) and 12-kDa, 20-kDa and 35-kDa PharmaPEGs, which have no methoxyl groups or other alkoxyl groups at the ends of the linear polymers, to be bound by anti-mPEG antibodies. Results are shown in FIG. 4. The shift of the three PharmaPEG curves relative to the mPEG curve depicted in FIG. 4 indicates that the antigenicity of PharmaPEG is approximately 100-fold lower than ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com