Method for the production of commercial nanoparticle and micro particle powders
a nanoparticle and powder technology, applied in the direction of biocide, grain treatment, drug composition, etc., can solve the problems of poor bioavailability, high dissolution rate, poor soluble active agent safety, etc., and achieve the effect of reducing static charge, reducing cohesiveness profile, and superior product flow characteristi
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Spex Milling
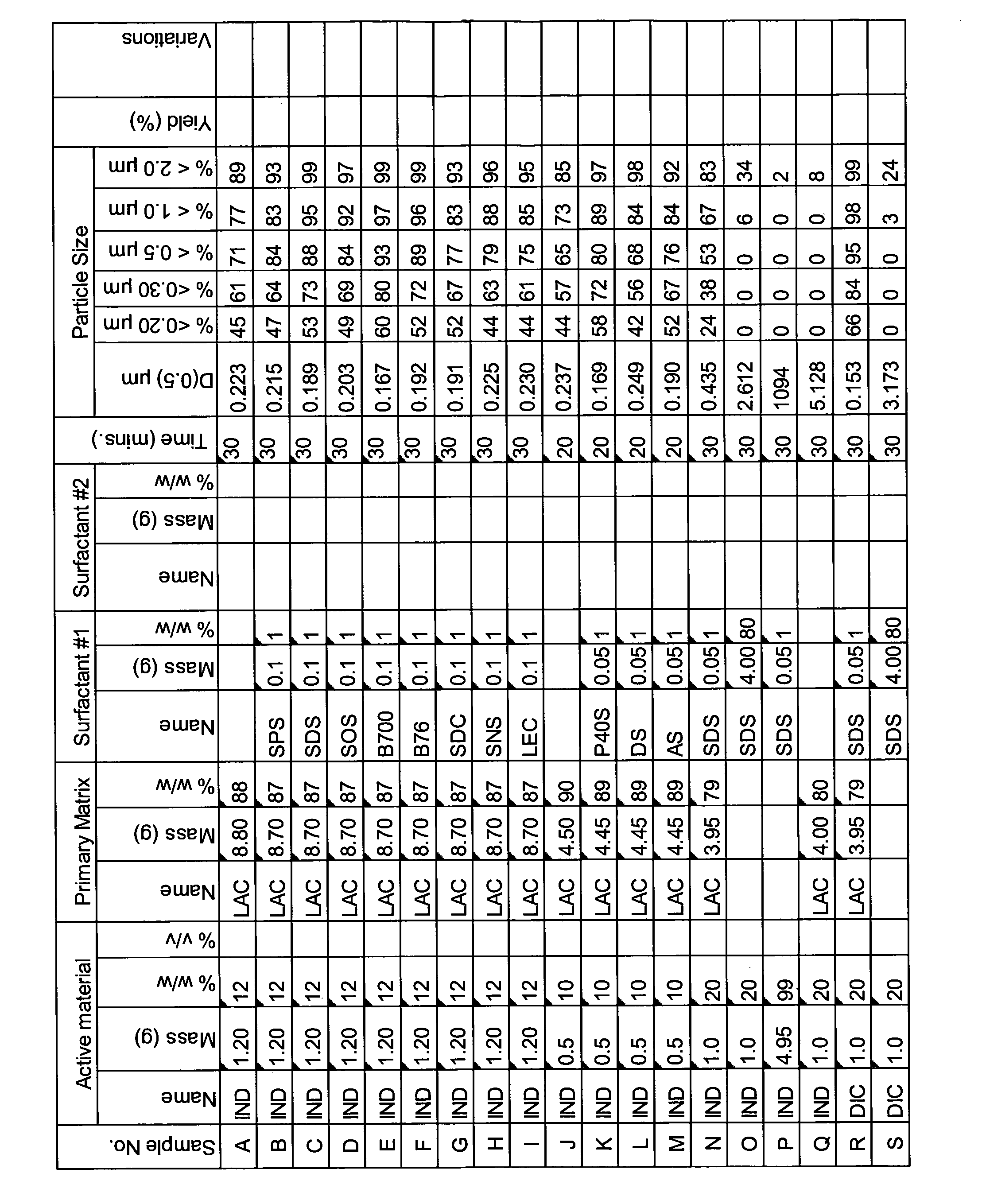
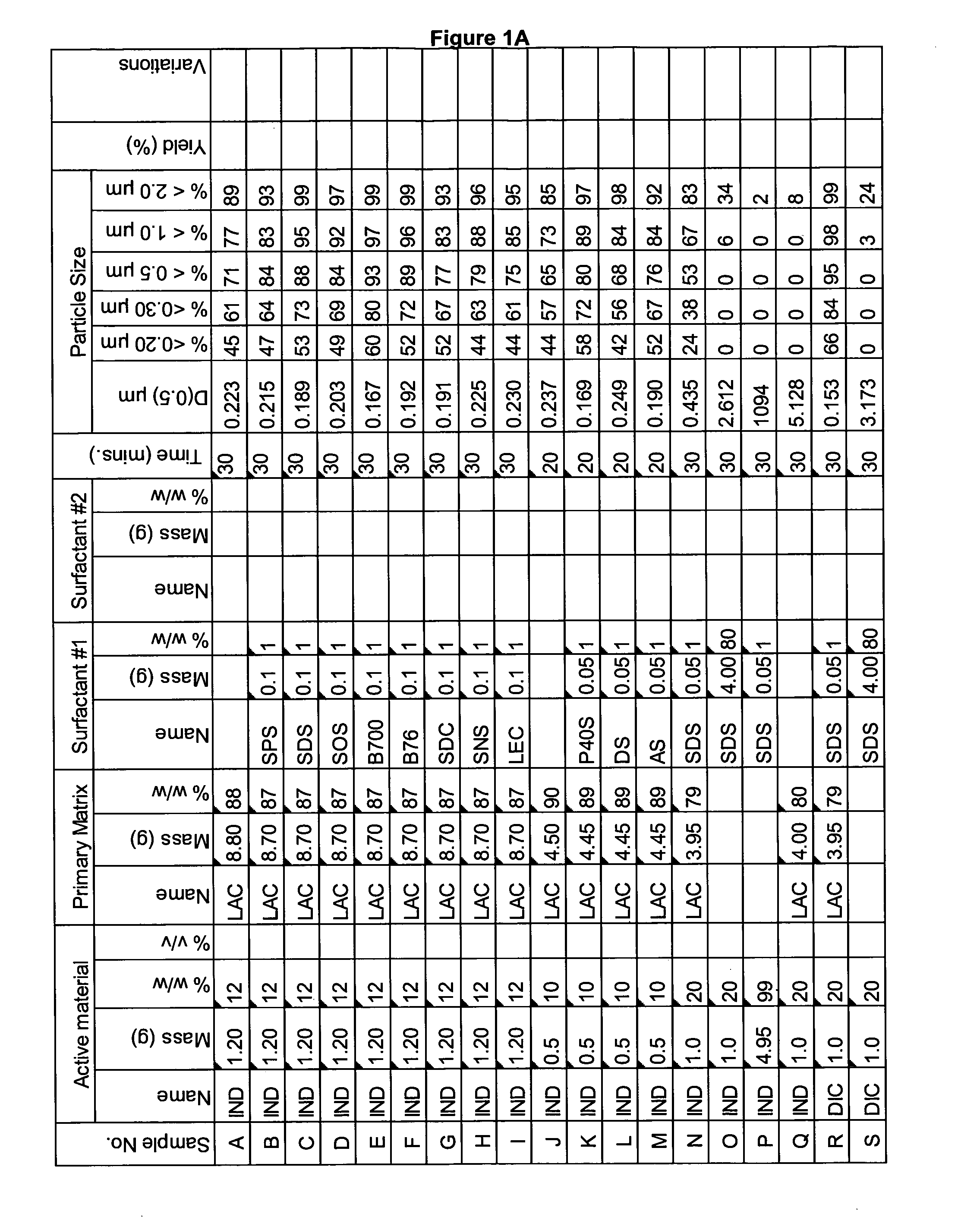
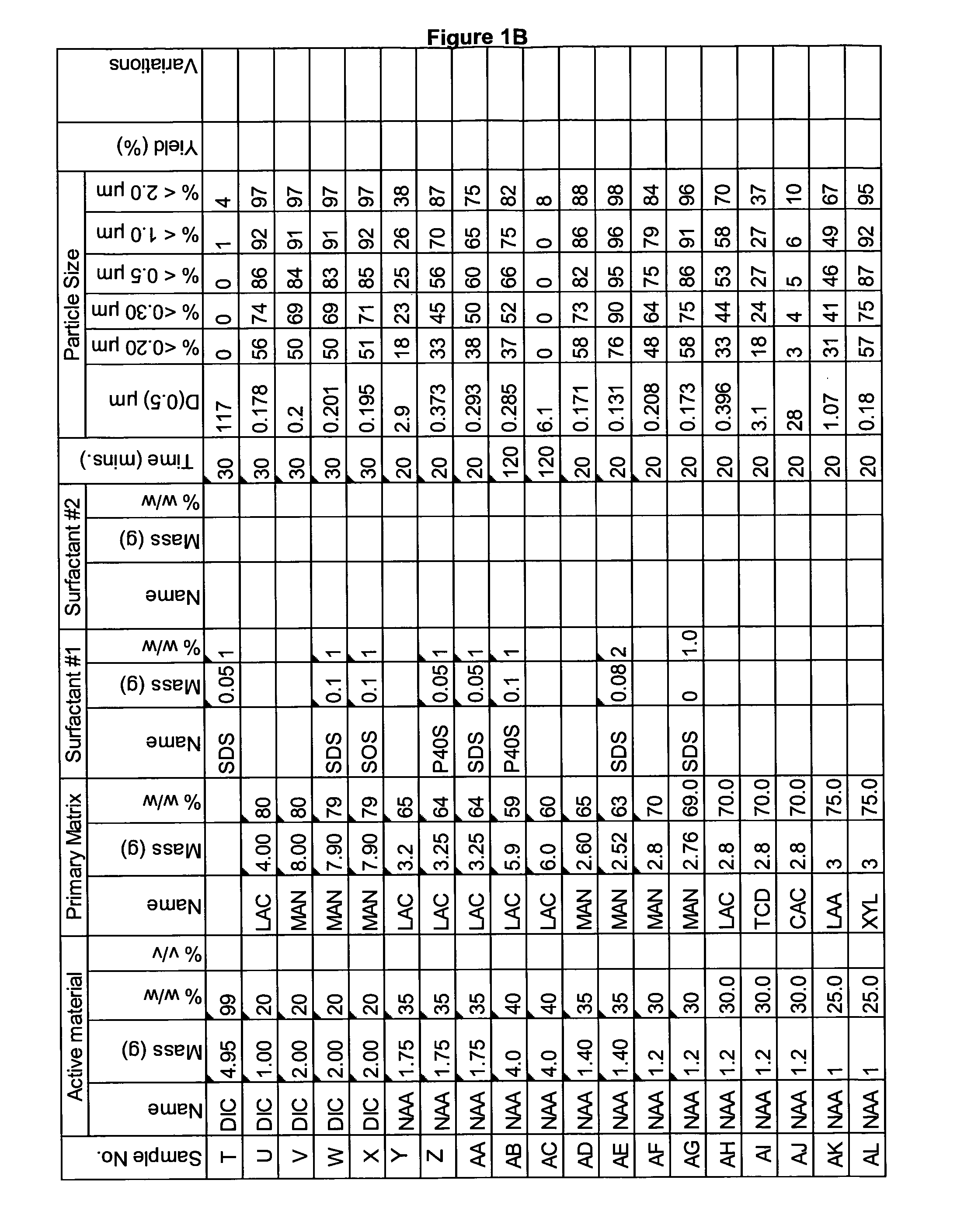
[0335]A range of actives, matrices and surfactants in a variety of combinations were milled using the Spex mill. The details of these millings are shown in FIGS. 1A-1G together with the particle size distributions of actives that were milled.
[0336]These millings demonstrate that the addition of a small amount of surfactant to the milling matrix delivers a smaller particle size compared to millings of just an active and a single matrix. Some examples of this are samples Z and AA compared to sample Y; Sample AB compared to sample AC; sample AE compared to sample AD; sample AG compared to sample AF; sample AP compared to sample AO; sample AR compared to sample AQ, sample AT compared to sample AS; Samples AX, AY and AZ compared to sample AW; sample BC compared to sample BD; sample BI compared to BH; samples BL-BR compared to sample BK; samples CS-DB compared to sample DC. This last example is particularly noteworthy as these millings were undertaken at 45% v / v. This demonstr...
example 2
110 mL Attritor
[0341]A range of actives, matrices and surfactants in a variety of combinations were milled using the 110 ml stirred attritor mill. The details of these millings are shown in FIG. 2A together with the particle size distributions of actives that were milled.
[0342]These millings also demonstrate that the addition of a small amount of surfactant to the milling matrix delivers a smaller particle size compared to millings of just an active and a single matrix in a small scale stirred mill as well as the vibratory Spex mill. Sample F also demonstrates that small particles can be achieved at high % actives when a surfactant is present. Sample D and E also show that the addition of the surfactant also increased the yield of powder from the mill.
example 3
Second Matrix
[0343]In this example naproxen was milled with a mixture of two matrices using the Spex mill. The details of these millings are shown in FIG. 3A together with the particle size distributions of actives that were milled. Samples A and B were milled in a primary matrix of lactose monohydrate and 20% of second matrix. The particle size of these millings is smaller than the same milling with just lactose monohydrate (See example 1 sample No AH, FIG. 1B). The particle size is also smaller than naproxen milled in the secondary matrices (See example 1 sample No AI and AJ, FIG. 1B). This shows the mixed matrices have synergy together.
[0344]Samples C-E were milled in anhydrous lactose with 20% of a second matrix. All these samples had a particle size much smaller than naproxen milled in anhydrous lactose alone (See example 1 sample No AK, FIG. 1B).
[0345]These millings demonstrate that the addition of a second matrix to the primary milling matrix delivers a smaller particle size ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com